Introduction
In the last article we were introduced to Mineral Synergism with respect to a third of the essential minerals.
In terms of the other 2 thirds, not much research has been completed, so we have no data concerning Synergism nor Antagonism for many trace minerals, although amounts required for the body are in microgram quantities, so we have to assume at this point that their absence is more important than their interaction.
Nutrient Relationships in Perspective

These 2 relationships between minerals also exist between Vitamins themselves, between Vitamins and minerals and even among hormones (even though this is regulated by the body).
Since my one theme throughout these articles is about biological balance to retain health, it is important to be aware of these nutrient relationships.
This is not to say that we need go through every nutrient and ensure balance by taking the correct amounts.
Apart from several specific nutrients such as ALA (Alpha Lipoic Acid), Ubiquinol, PQQ (Pyrroloquinoline Quinone) which are Anti-aging nutrients, the essential 90 nutrients are balanced by the body.
Throughout this series on essential nutrients I have provided a general list of foods that should contain particular nutrients, but we have no way of knowing if the food contains these nutrients unless you analyse them.
If the nutrients are not in the soil then the crop cannot absorb them, except for nutrient creation via photosynthesis.
In my series of articles ‘Paradise Lost’ I explain what has happened to many of the planets growing soils, depleted of natural soil nutrients and replaced with very few, i.e 60 minerals replaced with 3 minerals since this is the minimum to grow many nutritionless food crops.
This is why it is vital to supplement with all 90 essential nutrients harvested from land based ancient sea beds or the ocean itself which still contain the nutrients that all living organisms need to grow and thrive.
We must avoid these nutritional practitioners that behave like conventional doctors who match treatments with symptoms, instead of focusing on balancing the body as a whole
Having said that, if you suffer muscle cramps through exercise taking a little magnesium is a ‘no-brainer’, but if the nutritionist is faced with Fibromyalgia and not be aware of the true cause and then approach the patient as a regular physician would, your nutritionists office would become a revolving door experimenting with different individual nutrients until something improved.
Actual Multi-Faceted Mineral Relationships

Synergism and Antagonism toward nutrients occur for various reasons, Absorptive, Metabolic, Competitive, or even in the case of Substitution.
The absorption of one nutrient may impede the Absorption of another e.g A high intake of Calcium may suppress or reduce the absorption of Zinc, Magnesium, Manganese, Zinc or Potassium.
On the other hand the Antagonism may be related to Metabolism as in the case toward Zinc and Copper, Iron and copper, and Iron and zinc.
Although minerals may be Synergistic, an excessive intake of one mineral could create a deficiency in its Synergistic partner.
Such an imbalance can occur when excessive Zinc exists which may interfere with the Metabolism of Copper driving it to a deficient state.
Another consideration is that nutrients share ‘Binding Ligands‘ (e.g Oxygen is the ligand that binds to both Hemoglobin and Myoglobin), or
share a common ‘Receptor Site‘( e.g Receiver proteins that reside on the surface of each cell that perceive messenger molecules floating around in the bloodstream), or
Carrier Protein ( e.g these are proteins that carry substances from one side of a biological membrane to the other) and are therefore in competition with each other.
Typically, this competitiveness occurs between Copper and Zinc.
Another relationship exists between Iron and Copper whereby a toxicity level of Iron could occur in the presence or in this case absence of Copper (a deficiency).
Although Iron toxicity is relatively rare, it is more the case of deficiency (as in Anemia).
We mentioned Substitution, which in general is a favourable interaction, for example, an inadequate Vitamin E presence can be substituted by Selenium for the purpose of antioxidant activity.
Folate supplementation will temporarily prevent Anemia from B12 deficiency, but not the damage done by B12 deficiency. Since Tryptophan can be converted to Niacin, the body can substitute for it if there is a Niacin deficiency.
Vitamin C assists in Iron absorption, regulated by the body using natural Vitamin C from say citrus fruit, but taking a high dose Vitamin C supplement ( which also may be synthetic) may cause a Copper imbalance.
The USDA Western Human Nutrition Research Center at the University of California conducts nutritional studys in its quest to understand Human dietary requirements based on the wide variation of individuals response to diets, nutrients and other food constituents. It is this invaluable research that brings us closer to understanding this complex relationship between Minerals and Vitamins.
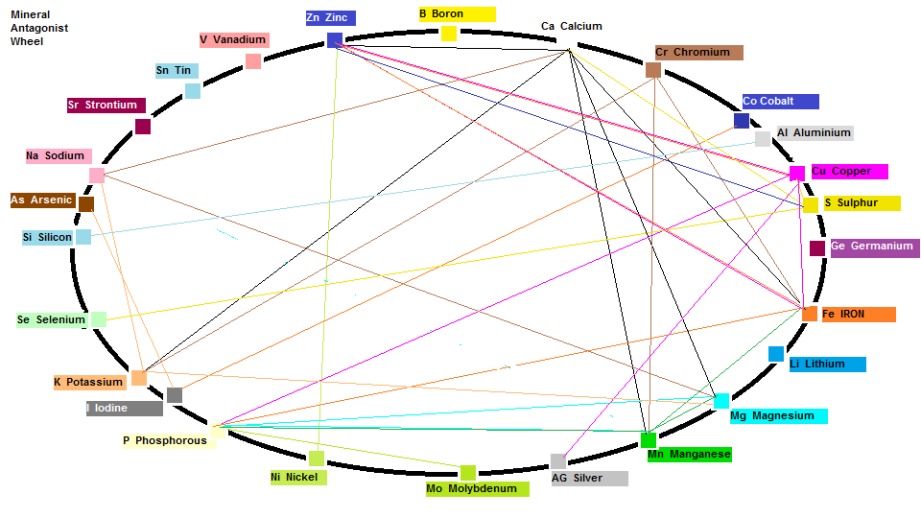
Mineral Antagonism that can cause Multi-Mineral Imbalance
If you study diagram 1 which displays Mineral Antagonism you will notice Antagonistic links between Manganese and Magnesium (On our synergistic chart in the previous article Magnesium needs Manganese but not the other way round).
In addition, there are Antagonistic links between Magnesium, Sodium and Potassium, so antagonistic effects that may occur between Manganese and Magnesium could result in excessive Potassium and Sodium accumulation for example.
Diagram 1: Mineral Antagonist Wheel
Nutrient Interaction and Bioavailability/Absorption (Zinc-Iron)
We mentioned the Iron and Zinc relationship in the previous paragraph, but let us examine a potential scenario.
It has been estimated that for Iron, males have a general storage of 1000mg reserve, and lose an average of 1 mg/day.
In females this reserve can vary between 200-400mg due to the menstrual cycle and their Iron loss could be between 1.5-2.4mg/day.
Zinc reserves for both men and women have been estimated to be in the range of 2-3 grams.
The daily loss of Zinc is estimated to be 2.3mg/day.
The current RDA for Zinc (Adjusted to 20-50mg/day) is equated, as in most nutrients, partially from absorption values which in the case of Zinc is approx 33% of intake amounting to around 15mg/day or 6.5 times the estimated daily loss, so compliance with the recommended intake (20-50mg/day) is more than enough to compensate for the loss at the higher value of 50mg/day.
In the case of Iron, whose RDA is 20mg, this mineral is available in 2 forms Heme and Nonheme, Heme Iron sources are meat and fish, Nonheme sources are plant derived.
However, absorption between the 2 is quite different, the estimated absorption for Heme Iron is approx 15-40%, and Nonheme is much lower at 1-15%.
Both forms of Iron are absorbed in Inverse Logarithmic proportion to the body’s reserve stores. This is expressed as:
Log Absorption = Mineral/Log Reserve store…BUT…
Phytic Acid (Phytate) inhibits Iron and Zinc absorption
So, for vegetarians, who rely on Whole grains, Legumes, Lentils and Nuts to derive their Iron intake should be aware that the Phytic Acid (also known as Phytate * the plant’s phosphorus storage used by young root sprouts for energy) content of these foods have an inhibitory effect on absorption, which is the same for Zinc as well.
Zinc’s absorption efficiency increases from aqueous sources and from animal products.
What can be done about this?.
The following strategies are used to reduce phytic acid:
- Soaking Legumes, whole grains, seeds, and nuts for 12-24 hours
- Fermented foods lower Phytic Acid due to the presence of Phytase ( an enzyme that degrades Phytic Acid) producing bacteria.
- Sprouted grains, such as sprouted grain bread have low Phytic Acid content as well as a ‘slow blow’ reaction to insulin ‘spikes’)
- Adding Vitamin C to meals has a Phytic Acid lowering effect as well as improving Iron absorption.
- If you have a balanced gut flora you have bacteria** that release Phytase that break down Phytic acid, with a value add, in that once these bacteria break down the phytic acid Phosphor is also released that the body can use.
*The supplement industry have even developed a supplement called Inositol Hexaphosphate, or IP6 which is a synthetic phytic acid supplement providing phytic acid ‘benefits’ that include phytic acid minerals binding in the gut preventing free radical formation (making it an ‘antioxidant’), heavy metals chelator, prevention of kidney stones from phytate excretion through the kidney, and prevention of Hemochromatosis (iron overload) by its iron binding action. Just to be aware of this ‘supplement’,
I personally would avoid it, you should be trying to reduce phytate for efficient nutrient absorption, so why would you want to put more phytate in your body when the supposed benefits can be realised by using other nutrients like Spirulina or Chlorella to chelate heavy metals, consume berries etc for antioxidant protection or boost your Glutathione and consume ginger on a regular basis which will support kidney function.
** The phytase producing bacteria include : Pseudomonas sp, Bacillus sp. Raoultella sp, Escherichia coli, Citrobacter braakii, Enterobacter, and anaerobic rumen bacteria, particularly Selenomonas ruminantium, Megasphaera elsdenii, Prevotella Sp.,Mitsuokella multi acidus, and Mitsuokella jalaludin.
The Heme/Nonheme Iron Relationship- ‘into the rabbit hole’

Biologically, Serum Ferritin is an indicator of Iron reserve stores. Ferritin is an Iron storage protein which resides in the Hepatocytes or liver cells and in Reticuloendothelial (RES) *** cells, and the body requests Iron to be released from either of these storage cells on an as needed basis.
Once released, it binds to another protein called Transferrin (our iron transport ‘truck’) which transfers it to the bloodstream.
***The cells of RES, which is also referred to as Mononuclear Phagocytic System (MPS). These systems consist of Macrophage ‘Clean up crews’ as well as Phagocyte defense against Pathogenic Mycobacteria, fungi, bacteria, protozoa, and viruses.
However, these systems are include Scavenger Endothelial Cells who are also part of the ‘Clean up crew’, but specifically target blood clearance by scooping up non functional Erythrocytes ( red blood cells), whereas the phagocyte ‘Janitors’ clear particulate matter such as bacteria, fungi, viruses, and dying cells from the circulation.
These ‘Janitorial Battalions‘ reside in the Adipose tissue (Fat), Bone Marrow/Blood( Scavenger Endothelial Cells are in abundance here), Liver, Lungs, Connective Tissue ( Collagen), Skin and Mucosa, Central Nervous system, Kidney, Bone and the Peritoneum ( represents the space or cavity where most of the organs are located inclduing the intestines, part of the rectum, pancreas, Kidneys, adrenal glands are located)
Inverse associations such as mentioned above:
Log Iron (heme/non heme) absorption = Mineral/Log reserve store (Serum ferritin)
This simple formulae suggests that there is a biological adaptation between Storage and Absorption, in other words the body’s iron stores are homeostatically regulated, and from studies, adaptation occurs with Nonheme dietary sources rather than from Heme sources.
In essence, the more Iron that is stored, the less that is absorbed, while Heme Iron accounts for nearly a half of iron absorbed in people with moderate iron stores, the up-regulation of nonheme iron absorption means that it is the Nonheme iron that contributes most to the Iron reserves when Iron stores are low (again…I have to remark how clever the body is to include a compensatory system to ensure that both types of iron are adequately bioavailable whether we are eating plants or meat ).
This fact only refutes the belief that vegetarians run a greater risk in becoming anemic, and conclusive results from studies show that iron deficiency Anemia is no more prevalent among vegetarians than meat eaters.

‘Even Deeper into the rabbit hole’ -Conclusion of our zinc/iron relationship
So to complete our Zinc/Iron story, when we speak of loss it can be interpreted as use, so storage of any nutrient will be depleted as the body uses that nutrient.
High Iron stores can be just as risky as being Iron deficient Anemic, since associations have been made toward colorectal cancer and reduced insulin sensitivity with large intakes of supplemental Iron.
There is also the issue of lower absorption efficiency associated with supplementation.
In fact the committee that dictate the Dietary reference intakes state that Iron Supplementation should be avoided.
Iron supplements are prescribed to pregnant women, but they also may need Zinc, so are their Iron and Zinc levels measured in order to balance their supplementation correctly ?..probably not, because your average allopathic physician is unaware of Antagonistic effects between nutrients, which means just prescribing a single nutrient without gathering pertinent Antagonistic data is not a good idea in my opinion.
It is possible that low Iron stores could increase the susceptibility of toxicity from other associated minerals like Copper and Zinc, but since Zinc’s synergistic minerals are Potassium, Magnesium, Manganese, Chromium and Phosphorous, all or either one could be affected by imbalances between Zinc and Iron.
Similarly Iron’s synergistic minerals are Copper, Manganese, Potassium, Sodium. Chromium, Phosphorous and Selenium.
Iron and Zinc’s common synergistic minerals are Chromium, Phosphorous, Potassium and Manganese which are more likely to be affected if an imbalance occurs in either Iron or Zinc.
An Example of Zinc deficiency-associated
Judith Turnlands paper on nutrient interaction recites a study that was performed using dietary Zinc, Sodium and Phytate, added for comparison purposes, and it clearly showed that adding Phytate to the mix reduces the absorption by 50% but we know this already.
What we don’t know is the condition of the gut flora of the test subjects which is an important factor.
We also know from our Antagonist chart that Calcium is a Zinc antagonist, however as Judith points out a diet high in Zinc,Calcium and Phytate would not result in a Zinc deficiency status, but if dietary Zinc was on the low side the combination of Calcium and Phytate would create a Zinc deficient situation.
Conclusion
So nutrient interaction affects bio availability either in a negative way or a positive way, that respectively inhibits or enhances nutrient absorption and/or utilization.
As we mentioned, these nutrient interactions occur between Vitamins and Minerals which we will discuss in a subsequent article.
Having read the contents of this article it is clear that the correct balance of nutrients and food for the body is complex and if corporations and industry had not tampered with the human food chain and left the soils rich with nutrients it would not be necessary to go through this analysis.
The body possesses the blueprints for our food and the body can deal with nutrient balance, and as we have seen, most nutrients are homeostatically regulated and any excess is expelled from the body …. so all we had to do is eat, supplement and live a long and healthy life.
“As mineralogy constitutes a part of chemistry, it is clear that this arrangement [of minerals] must derive its principles from chemistry. The most perfect mode of arrangement would certainly be to allow bodies to follow each other according to the order of their electrochemical properties, from the most electro-negative, oxygen, to the most electropositive, potassium; and to place every compound body according to its most electropositive ingredient.”
-Jöns Jacob Berzelius
An Attempt to Establish a Pure Scientific System of Mineralogy (1814)
Check out the Previous Article in this series:
https://www.extremehealthacademy.com/90-essential-nutrients-part-1-overview/
https://www.extremehealthacademy.com/90-essential-nutrients-part-2-b-vitamins-1-6/
https://www.extremehealthacademy.com/90-essential-nutrients-part-3-b-vitamins-7-12/
https://www.extremehealthacademy.com/90-essential-nutrients-part-4-vitamins-acde/
https://www.extremehealthacademy.com/90-essential-nutrients-vitamin-d/
https://www.extremehealthacademy.com/essential-nutrients-vitamin-e/
https://www.extremehealthacademy.com/essential-nutrients-vitamin-k/
https://www.extremehealthacademy.com/essential-nutrients-choline/
https://www.extremehealthacademy.com/essential-nutrients-part-9-flavonoidspolyphenols/
https://www.extremehealthacademy.com/essential-nutrients-part-10-amino-acids/
References/Acknowledgments:
- Minerals for the genetic code Charles Waters/Dr Richard Olree 2013
- Adaptation of iron absorption in men consuming diets with high and low iron availability 1,2,3,4 J.Hunt & Z.Roughhead 2000 American Society for Clinical nutrition
- Modern Nutrition in health and disease M.Shils, M.Shike, C.Ross, B.Caballero, R.Cousins
- Epigenetics Essential minerals section Joel Wallach book 2014
- Nutrient interaction issues Judith Turnlund
- Nutrient interrelationships Minerals, Vitamins, Endocrines David Watts
- Ferritin level blood test Rachel Nall 2015 Healthline
- Phytates and Phytic acid Precisionnutrition.com
- Bacterial phytase: potential applications. In vivo function and regulation of its synthesis U. Konietzny, R.Geiner Brazilian journal of microbiology
- Reticuloendothelial system (RES), Mononuclear Phagocytic system (MPS), Scavenger Endothelial cell (SEC). Wikipedia
- Quotes TODAYINSCI Website
Author: Eric Malouin