Introduction
Vitamin E complex consists of 4 Tocopherols (α, β, γ, δ forms) and 4 Tocotrienols (α, β, γ, δ forms).
Since it is a fat soluble Vitamin the Tocopherols a saturated fat side chain (Fats or Triglycerides are made up of glycerol molecule attached to 3 fatty acid side chains which determines the type of triglyceride) which would resemble the consistency of Butter or lard that are solid at room temperature and in the refrigerator.
Tocotrienols contain Polyunsaturated Fat (PUFA) such as in nuts, or a consistency of a vegetable oil which will not solidify unless it is cold (I keep a little olive oil in my garage that solidifies partially at around -15).
Vitamin E is used in an antioxidant capacity in all cells in partnership with Vitamin C (Ascorbate) ,Glutathione, Alpha Lipoic Acid (ALA), Coenzyme Q10 (Ubiquinone) and NAD/NADH/NADPH, which are all constituent parts of an ‘Antioxidant Network’.
Vitamin E is used as an antioxidant by the liver to protect its cholesterol from oxidation during transport within a lipid raft (cholesterol boat) called Low density lipid protein (LDL) to various parts of the body.
In this process the liver also use another cholesterol transport High density lipid protein (HDL) which is the return journey back to the liver containing the ‘scooped up’ unused Cholesterol which is then recycled within the Bile salts.
Finally Vitamin E is also featured in an antioxidant capacity within the Epidermis of the skin.
Reactive Oxygen Species (ROS)
So why do we need antioxidants?
During various normal biological processes such as energy production and metabolism these processes create waste products (Metabolites), similiar to exhaust from an automobile.
These products occur as Reactive Oxygen Species (ROS) which include free radical substances that have become oxidised from its interaction with oxygen which can lead to ‘Oxidative Stress’.
Free radicals, Lipid peroxides, and Heavy Metals are all Reactive Oxygen Species (ROS) , substances missing an electron making them very unstable.
The substance which has lost an electron becomes an unstable free radical and begins its quest to establish chemical balance by attempting to steal an electron from some other molecule.
By stealing an electron to stabilize itself, ends up damaging the non-consenting donor, and once this occurs a cascade effect commences which can easily get out of control, causing widespread damage and ultimately oxidative stress which can cause dis-ease to erupt.
Antioxidants
The purpose of an antioxidant is to give up one its electrons in order to neutralise the free radical, but making this ‘sacrifice’ it to becomes a free radical itself (antioxidant radical),but since this is part of its design, it relies on another antioxidant to reduce or re-active it back to its antioxidant state. This process is known as Redox (Reduction-Oxidation reaction):
- Oxidation (LOSS of an electron)
- Reduction (GAIN an electron)
Examples of the Redox process is Rust on metal which is a Slow Redox process, while a Burning Fire is a Fast Redox process.
On a positive note, defensive cells of the immune system create free radicals purposely to neutralise pathogenic viruses and bacteria, but this is one of very few scenarios where the body can utilize free radicals to its advantage, but normally free radicals need to be neutralized to mitigate tissue and organ damage.
The Body’s Own Natural Antioxidant:
Glutathione (GSH)
The body manufactures its own powerful antioxidant called Glutathione (GSH), a sulphur based molecule made from 3 amino acids L-Cysteine, Glycine and L-Glutamic acid.
Glutathione is not an essential nutrient nor are the amino acids that make it because this substance can be sythesized in the body, and all 3 amino acids can be found in Meat (Turkey, chicken, beef, lamb), Sunflower Seeds, Fermented soybean products e.g Natto which contains approx 63mg/100 g of cysteine and Tempeh that contains approx 60 mg/100g, Oats, Fish and Eggs.
I would not recommend taking a Glutathione Supplement since, I believe the body cannot use it in its pre-built form, but to consume the building blocks (the 3 amino acids) which the body can use to create Glutathione.
As we age Glutathione production diminishes, and you may not be consuming enough of the 3 amino acid foods, so I would recommend consuming food that contains a complete Amino Acid profiles such as Organic Whey powder for example.
Note: I would recommend not to take Whey powder ISOLATE, since it is high heat treated, acid flushed and stripped of nutrients and some contain unwanted chemicals and additives and quite frankly Acidifies the body.
Biological Function:
Cholesterol Oxidation Protection in LDL
Vitamin E is a true antioxidant and a very powerful and essential nutrient.
Vitamin E is a major lipid soluble antioxidant in Biomembranes and one of the ‘vitamins of choice’ by the liver that includes it when packaging a Low density Lipoprotein (LDL) for cholesterol transport.
The LDL is miniscule in size measuring approx 220-275 Angstroms (1 Angstrom = 0.1 nanometre or 10 raised to the power of -10 = .00000000001 metres) in diameter, with a molecular weight of 385 g/mol ( a water molecule weighs 18 g/mol for comparison purposes).
The LDL Cholesterol transport particle contains a variety of antioxidants which are listed in the Table 1 below:.
Table 1
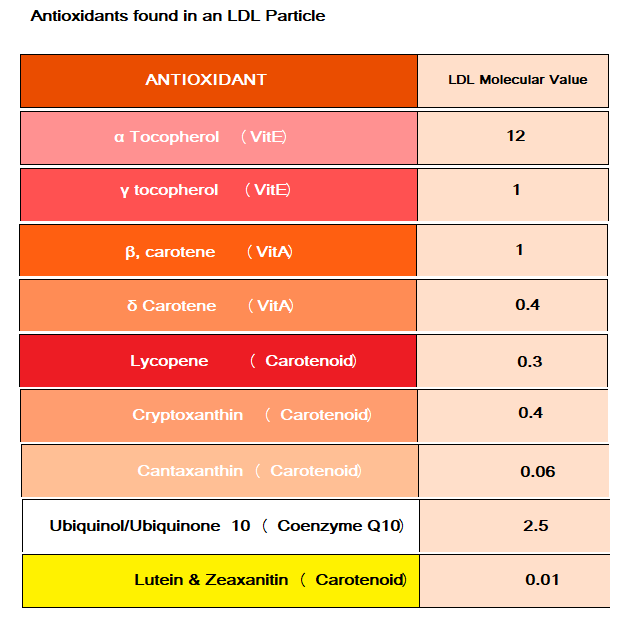
As you can see from Table 1, α Tocopherol (Vitamin E) is by far the most abundant antioxidant that the liver uses to protects its cholesterol within an LDL particle. The LDL particle contains Cholesterol, Cholesterol Esters, Phospholipids, Triglycerides, 1 APO B Protein (coded from gene APOB and is basically an ‘Address Tag’ that mediates the interaction with target molecules and cells – acts like a GPS to find the cell that needs the cholesterol) and the various Antioxidants from Table 1 is shown in Diagram 1
Diagram 1: LDL Particle
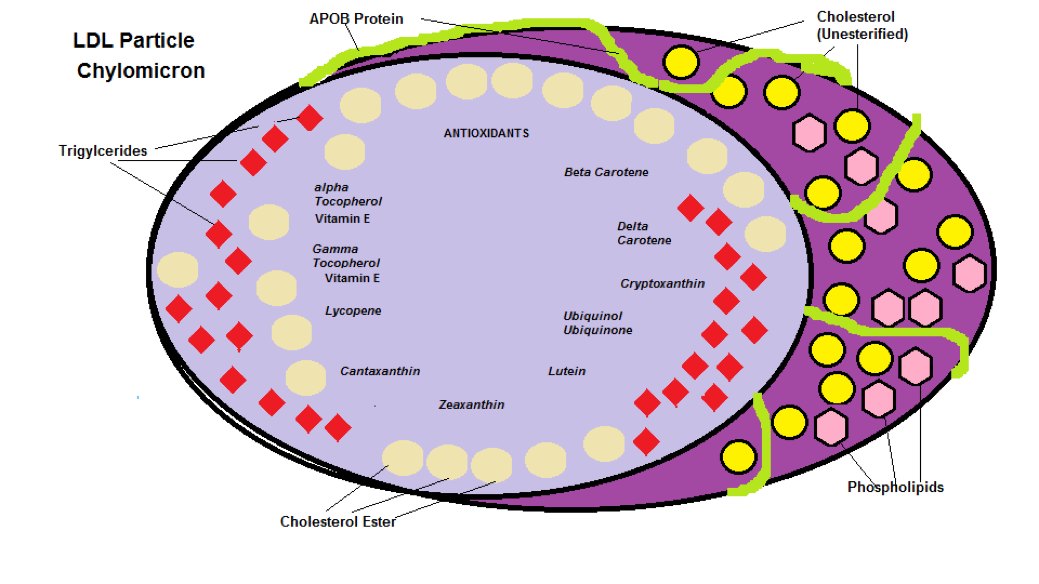
Free Cholesterol vs Cholesterol Ester
When the LDL particle reaches its destination, the LDL transfers its cargo into the cell via a process called Endocytosis (there are 4 endocytosis pathways that we will not discuss here), a process that also occurs during the uptake of cholesterol by intestinal Epithelial cells.
Cells will only accept ‘Unesterified Cholesterol’ or Free Cholesterol (this is the active form of cholesterol), as opposed to the storage form of Cholesterol known as ‘Esterified’ or ‘Cholesterol Esters’.
Only ‘Unesterified Cholesterol’ is absorbed from the diet, since the Ester form is too bulky.
The liver actually uses a distribution mechanism called the Hepatic Alpha Tocopherol Transfer Protein for distribution throughout the body via VLDL (Very Low density proteins) which gets converted to LDL.
In Diagram 1 the ratio between Cholesterol Esters and Triglycerides is 4:1
The Unesterified Cholesterol on the outer surface alongside the APOB Protein is there for structural purposes to maintain integrity of the particle.
The Antioxidant Network within the cellular Mitochondria
To protect the cell, Vitamin E leads the protective charge with its other antioxidant partners Glutathione Vitamin C, Coenzyme Q10 ,Alpha Lipoic acid and NADH/NADPH as shown in Diagram 2. The network acts as a recycling factory for each other since the process of antioxidant activity is to neutralise free radicals that are produced from normal metabolism and energy production.
We all need to breathe oxygen due to our innate design and many processes use an aerobic environment, so oxygen is life, but not without consequences, including oxidative stress, and in many older folks who do not help the body to combat oxidation, and their normal antioxidant capability diminishes they become sick and many bear the scars such as Lipofuscin on the skin (face and hands).
Conventional physicians call these brown spots ‘Liver spots’ or Age spots’ but in reality they are signs of oxidative stress and excessive un-quenched free radicals occurring inside the body.
When we are younger our own free radical ‘buster’ which is the body’s most powerful antioxidant called Glutathione takes care of everything, but as we age Glutathione production diminishes and these spots begin to show up in abundance.
Endogenous oxidants arise from normal natural processes like metabolism.
During cellular respiration oxidant substances such as Superoxide and Hydrogen Peroxide are produced from Lipoxygenase, Xanthine oxidase and NADPH oxidase.
In addition to Exogenous oxidants from diet, UV radiation and inhaled chemical contaminants will all increase free radical production that need to be quenched.
Diagram 2 shows the protective antioxidant regeneration pathways.
Diagram 2: Antioxidant network within the cellular mitochondria
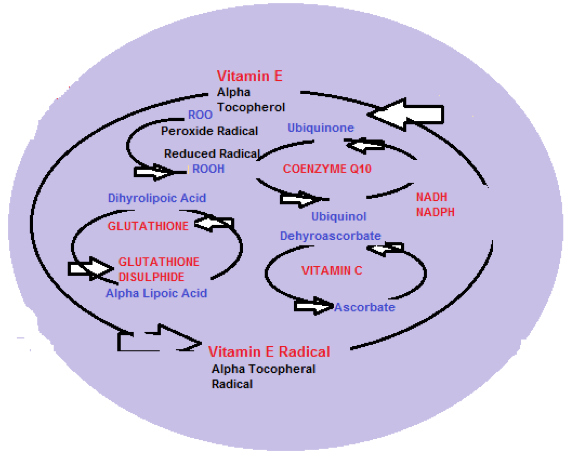
Vitamin E/Vitamin C: Antioxidant Relationship
The role of Vitamin E (alpha Tocopherol) within the mitochondria is to convert alpha Tocopherol to alpha Tocopherol radical as an antioxidant reaction, by donating a labile (easily changed) hydrogen molecule to a Lipid peroxyl radical (ROO) and the alpha tocopherol radical is then reduced (converted) back to alpha tocopherol by Ascorbic acid (Vitamin C).
ROO + TOH ————- ROOH + TO (alpha tocopherol donates Hydrogen producing alpha tocopherol radical)
Ascorbate + TO———–Ascorbate +TOH (TO is then recycled by ascorbate and enzymes detoxify and recycle ascorbate radical (Dehydroascorbate) back to ascorbate.
Glutathione and Alpha Lipoic acid Relationship
Once Glutathione does its job as an antioxidant and donates an electron, it to becomes oxidised and becomes Glutathione Disulphide (GSSG). Alpha Lipoic Acid (ALA), not to be confused with Alpha Linoleic Acid the Omega 3 fatty acid, is another antioxidant network partner that can reconstitutes
Glutathione from GSSG back to its active (reduced) form GSH by an interaction with an enzyme Gamma Glutamylcysteine ligase involved in Glutathione synthesis. This ALA-GSH antioxidant recycling pathway is shown in diagram 2.
In fact, ALA is both water and fat soluble, making it a very flexible antioxidant that can scavenge reactive oxygen and nitrogen species throughout the body.
ALA also is capable of recycling the other partners of the antioxidant network such as Vitamin E,C and Coenzyme Q10.
ALA is also used as chelator for heavy metals in the body.
Tiny amounts of ALA are contained in Broccoli, spinach, potatoes, brewer’s yeast, liver, red meat.
Since both ALA and DHLA (Dehyrolipoic Acid)both possess antioxidant capability, that can scavenge free radicals, perform metal chelation and have antioxidant recycling ability.
Since DHLA is more potent as an antioxidant it works together with Glutathione, Ascorbate and Tocopherol.
NAD/NADH/NADPH
Nicotinamide Adenine Dinucleotide (NAD) is the cofactor (or Coenzyme) for Niacin.
If you have read Part 1 of this series you may remember that NAD is derived from the Amino acid Tryptophan, that also relys on Riboflavin (FAD) for its synthesis and NAD is used for all Fuel – Energy conversions for cellular energy production.
In addition, FAD is also used to synthesise the body’s most powerful antioxidant Glutathione in conjunction with the enzyme Glutathione reductase. Both NADH and NADPH are important electron carriers during the antioxidant activity of electron exchange.
The role of NADH is used in Catabolic reactions (from previous articles we explained that Catabolism is the breaking down of substances to release energy) while NADPH is used for Anabolic reactions (Anabolism is the formation of biological building blocks like proteins and nucleic acids).
In terms of Antioxidant activity, NAD is the Oxidised form, while NADH is the Reduced form.
Similarly NADP is the Oxidised form, while NADPH is the Reduced form.
The primary task from the perspective of the cell is to generate energy, so the NAD/NADH ratio that Releases Energy is kept High while the NADP/NADPH ratio that Consumes Energy is kept Low.
Coenzyme Q10 (CoQ10)
Coenzyme Q10 or as it is also known Ubiquinone which in ‘antioxidant speak’ is the Oxidised or Inactive form while Ubiquinol is the Reduced or Active form.
Ubiquinone (so named because it is found everywhere in the body with the highest concentration in the heart since it is the main antioxidant for the heart cells).
Within the Mitochondria, it serves 2 main functions, as an antioxidant, and its role as a one electron transport mechanism for the tail end of the Energy cycle (Krebs) of the Electron Transport Chain.
To release energy to generate ATP for the electron transport chain within the mitochondria, our cells, through an Oxidative Phosphorylation Metabolic Pathway, use enzymes to oxidise nutrients.
This process involves electron transfer from electron donors to electron acceptors in the same way as in the Redox process of antioxidants, so Coenzyme Q10 is also a perfect antioxidant, in the same manner as the other lipid soluble antioxidants partners within the Antioxidant Network.
Coenzyme Q10 as an antioxidant uses a redox active group called Benzoquinol.
It is interesting to note here, that the Pharmaceutical drug that lowers cholesterol called Statins block the biological pathway (Mevalonate pathway) inhibiting cholesterol transfer.
Having said that, our liver only synthesizes about 20% of cholesterol (80% is made by our cells) so when the ignorant medical world measure plasma cholesterol it has little to do with cellular cholesterol and above all arterial cholesterol, because nobody knows the destination of the cholesterol molecules (NOW you can understand what a farce it is, and indeed based on a false premise to prescribe cholesterol lowering drugs).
CoQ10 is produced primarily in the liver and then converted to Ubiquinol in the body through an enzymatic Redox cycle as we explained earlier.
CoQ10 must be “reduced”into its Ubiquinol active form before it can be used in the body.
The Mevalonate pathway is shared between cholesterol and Coenzyme Q10 so both products are slowed down, and ‘so we all jump on the mythical medical theory that Statins lower cholesterol which protects your heart’.
This is pure tosh and I have to laugh at the news that in 2014 the Cochrane collaboration meta analysis found no evidence to support or refute the use of Coenzyme Q10 for the treatment of heart failure..really, then tell me why the biggest concentration of CoQ10 is in the heart..for storage perhaps..somewhere to put it temporarily..the medical profession must think we are dumber than cows.
Unless you supplement with CoQ10, if you take statin drugs, you are starving the body of a crucial antioxidant and instead of protecting your heart you are putting it at risk. We cannot forget the function of cell membrane Caveolae within the Pacemaker cells (Nodal cells) of the heart that was described in the previous article, where, if cholesterol is restricted, this can compromise the cardiac rythmn of the heart causing heart arrythmia and even heart failure.
Finally, CoQ10 works in conjunction with Vitamin E (Alpha Tocopherol, α-TOH), so when α-TOH neutralises a free radical it becomes oxidized itself as explained earlier forming α-TO.
When Ubiquinol reacts with α-TO it reduces it back to its non-oxidised or active form α-TOH.

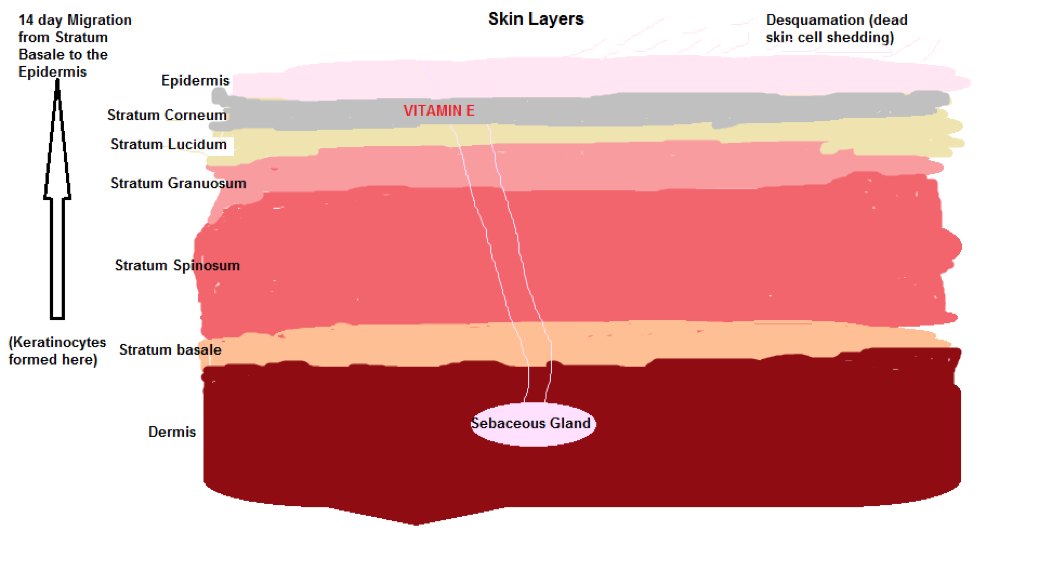
Vitamin E and the Skin
Vitamin E is found in various parts of the skin, the Dermis, the Epidermis and the Stratum Corneum and acts as an antioxidant protecting the skin from oxidative stress.
The most abundant form of Vitamin E Alpha tocopherol exists in the skin layers.
VitE is stored in Keratinocytes (outer layer skin or epidermis cells). These Keratinocytes are formed in the Stratum Basale of the skin and follow a 14 day migratory path from the Stratum Basale to the outer skin surface where they are are subjected to Desquamation (fancy word for the peeling off of dead skin ) which is the normal skin cell replacement cycle.
VitE is also secreted by Sebaceous glands (SC), microscopic Exocrine glands that lubricate and waterproof the skin…I told you the body was smart..you don’t even need to wear a raincoat in the rain cause you have natural waterproofing).
One of the Sebaceous glands rich regions is the face where the skin is thinner.
This skin lubrication begins to diminish in women over 50-59 and in men older than 70 ( that sucks since women need their facial looks more so than men so why should women’s SC’s decline earlier than men ?).
Diagram 3 shows the various skin layers:
Diagram 3 Skin Layers
Vitamin E Bioavailability
Vitamin E for me has always been a little enigmatic, since it is a much needed antioxidant to protect cells and to recharge antioxidant capability for other antioxidant substances, and its protection of the skin (the largest organ is the skin).
Yet its availability in the diet is sparse, and its absorption requires bile acids ( good luck absorbing Vitamin E if the white coat marauders have conducted an abscission on your gall bladder).
Furthermore, Vitamin E extraction also requires Pancreatic Esterase to release the fatty acids from dietary triglycerides, and a hydrolytic cleavage of Tocopherol Esters which are the common form of dietary Vitamin E supplements.
In addition, once any form of Vitamin E has been absorbed it is packed up into a molecule transport called a Chylomicron (Lipid protein) containing Vitamin E, dietary fats and cholesterol and sent to the liver which uses only alpha tocopherol and excretes all other forms (WHY ?).

Vitamin E Food Sources
Nuts,seeds and vegetable oils appear to be the richest sources that contain Vitamin E, but avoid the vegetable oils because they are damaged oils from the high heat processing they use to make the stuff.
- Wheat germ (this is the nutrient package that is removed from the wheat when making bread, otherwise the bread won’t be as smooth and white and nutritionless) oil ( 1 Tbsp = 20mg )
- 1 ounce of Almonds = 6.8mg
- 1 ounce of Brazil nuts = 1.6mg
- 2 Tbsp of Peanuts = 2.2mg
- 2 Tbsp of Peanut butter = 2.9mg
- 1/2 cup of spinach = 1.9mg
- 1/2 cup of brocolli = 1.2mg
- 1 Kiwifruit = 1.1mg
- 1/2 cup Mango =0.7mg
- 1/2 a Sapote fruit = 5.9mg
- 1 med Tomato =0.7mg
- 100g Sunflower seeds = 36mg
- 100g Rainbow trout = 2.8mg
- Half filet( 200mg)Atlantic Salmon = 2 mg
- 1/2 an Avocado = 2 mg
- 1 medium Pepper = 1.9mg
- 1 ounce Pumpkin seeds = 0.6mg
- Green Leafy vegetables also contain an amount of Vitamin E
So you see from the list, apart from wheat germ oil (wheat germ powder only has approx 2 mg/Tbsp but it contains a good amount of zinc), the amounts in most foods are meagre so it might be best to supplement.
The other problem is that the poisonous oils which you are going to avoid anyway contain Gamma Tocopherol which the liver excretes, in addition how much alpha tocopherol vs the other VitE forms are contained in the food listed above.
If you supplement, try and find a water dispersible succinate form of VitE ( preferably in a whole food supplement such as ‘Supergreens’ from LivingFuel) which is usually in powder form, since it absorbs well, and avoid any pill forms of VitE, since the amounts of the vitamin contained therein are negligible and absorption is questionable.
Always take Vitamin E with fat to enhance absorption.
Do not exceed 15 mg/day since it has an antagonistic relationship with Vitamin K.
An important dietary fat is Polyunsaturated Fat (PUFA) especially to supply the body with Omega 3/6 fats which are PUFAs and critical for the manufacture of Prostaglandins (Eicosanoids).
In Part 3 of the series Metabolic typing, we discussed Prostaglandins in detail but to refresh, these messenger molecules behave in a similar way as hormones ( endocrine derived messengers used to stimulate cells/tissues to function ) and are involved in inflammation response, and promotion to treat injury and effect repair.
They also regulate human reproduction and induce labor, and protecting the heart by providing anti-clotting and the management of excess triglycerides ( fats in the blood) and blood pressure control.
To avoid compromising these vital systems the Liver protects the PUFAs using Vitamin E Alpha Tocopherol ( α-TOH ) from Lipid peroxidation, during transport ( from the initial absorption site, the epithelial enterocytes (Chylomicron Transportation) and the Lipoprotein distribution rafts (VLDL Transportation)) within the LDL particle and its final destination within cellular membranes and red blood cells.
We know that the liver uses an α Tocopherol transfer protein (α-TPP) to preferentially transfer α Tocopherol vs other forms of Vitamin E to the Hepatic plasma membrane, where the ATP cassette transport that is involved in the transfer to circulating lipoproteins (VLDL).
From the study in 2013 by Maret Traber ‘ Mechanisms for the prevention of Vitamin E excess’ estimates that VLDL transports contain 65 α Tocopherol molecules, LDL transports contain 8-12 α Tocopherol molecules, and HDL contains < 1 α Tocopherol molecules.
Despite the fact that plants synthesize 8 different form of Vitamin E (4 Tocopherols (α, β, γ, δ forms) and 4 Tocotrienols (α, β, γ, δ forms), the liver only prefers to use α Tocopherol. and excretes everything else
Conclusion
I read somewhere that one or more members of the VitE family may reduce ageing, protects the nervous system and retina, lowers the risk of coronary heart disease, and reduces the risk of alzheimer’s.
How does it achieve these great things ?, since it is not one or more members, it’s only one family member alpha tocopherol since we have already established that the liver excretes all other forms, and even if it did not, the other forms are less potent e.g Gamma Tocopherol has between 10-30% potency compared to alpha tocopherol.
As we have established earlier, it is CoQ10 which prevents heart disease and its the VitE component that regenerates it once it becomes oxidized from performing its antioxidant function.
My last word, or it’s more of a question, most research articles claim that VitE deficiency is rare and yet given the specific absorption requirements, the narrow food sources that contain VitE, and the very few who supplement, begs the question.
Why ?..Do you know?
Well, if you have read this article with the same passion as its writer…lol you will know the answer (which incidentally is not answered in most texts).
It is because of the marvelous antioxidant network where nutrients are recycled and regenerated
(I will say it again..we host a very, very smart body).

“Men occasionally stumble over the truth, but most of them pick themselves up and hurry off as if nothing ever happened.”
–Winston Churchill
“In the course of my life, I have often had to eat my words, and I must confess that I have always found it a wholesome diet.”
–Winston Churchill
Check out the Previous Article in this series:
https://www.extremehealthacademy.com/90-essential-nutrients-part-1-overview/
https://www.extremehealthacademy.com/90-essential-nutrients-part-2-b-vitamins-1-6/
https://www.extremehealthacademy.com/90-essential-nutrients-part-3-b-vitamins-7-12/
https://www.extremehealthacademy.com/90-essential-nutrients-part-4-vitamins-acde/
https://www.extremehealthacademy.com/90-essential-nutrients-vitamin-d/
References/Acknowledgments:
- Antioxidants for health and longevity Dr Lester Packer
- Vitamin E and the antioxidant network: Protection of LDL from oxidation Dr Lester Packer SpringerLink Website
- LDL Wikipedia
- Free Radicals,Oxidants and Antioxidants G.Buettner,F.Schafer 2000 Teratology website
- Alpha Lipoic acid benefits Immunehealthscience website
- Modern Nutrition in health and disease M.Shils, M.Shike, C.Ross, B.Cabellero,R.Cousins
- Investigating mitochondrial redox state using NADH & NADPH autofluorescence T.Blaeker, M.Duchen NCBI 2016
- Handbook of cosmetics science and technology A.Bariel, M.Paye, H.Maibach, Book 2010
- Vitamin E NIH website
- Top 10 foods highest in Vitamin E you can’t miss HealthaliciousNess website
- The straight dope on Cholesterol personal blog from Peter Attia MD
- ‘ Mechanisms for the prevention of Vitamin E excess’ 2013 by Maret Traber NCBI (PubMed)
Author: Eric Malouin