Table 1: Essential Amino Acid Profiles

Introduction
We as humans biologically would not exist without Amino Acids which are the building blocks of life itself.
Amino Acids are made from Carbon,Hydrogen,Oxygen,Nitrogen and (some) Sulphur.
When the body requires protein synthesis it unwinds and reads its blueprint of genetic instructions housed within our double helix Deoxyribonucleic Acid (DNA), makes a copy and produces a messenger Ribonucleic Acid (mRNA).
The unwound DNA is rewound and the body uses the mRNA to produce the protein consisting of one or more long chains of amino acid residues not unlike a strand of beads.
The amino acid sequence that make the protein is a gene sequence encoded in the genetic code contained within the blueprint copy, the mRNA.
The genetic code makes use of some 20 amino acids, 12 of which ( some say 9) are Essential or Indispensable, that have to enter the body from dietary intake, since no biological pathways exist for them, so the body cannot make them.
The remaining 8 (or 13) are Non-essential or Dispensable since they can be manufactured in the body.
The particular amino acid sequence distinguishes each protein and its corresponding fold pattern into a 3 dimensional structure that defines its activity.
It is the chemical properties of amino acids that make the protein determine the biological function of the protein.
Proteins control all cellular activity.
The amino acid sequence is bonded together by Peptide Bonds.
Peptide bonds are covalent chemical bonds ( the sharing of electron pairs between atoms (e.g Hydrogen H2 share the 2 atoms via covalent bonding)).
Amino Acid Structure
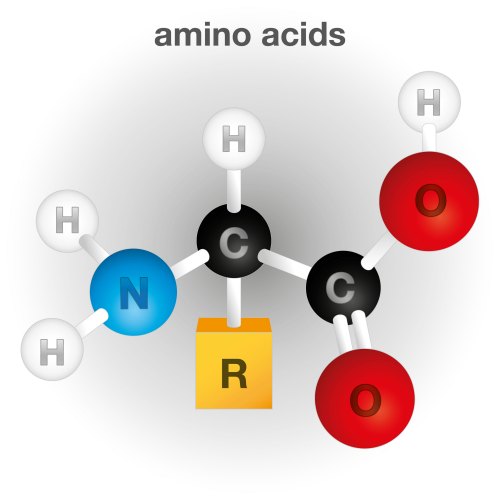
Proteins can have thousands of Amino Acids, where each amino acid consist of a Carboxyl-Carbon Group (Carboxylic Acid COOH) and an Amino-Nitrogen (NH2) group attached to a Central Alpha Carbon including specific side chain elements as shown abo,e. and are classified into functional groups as displayed in Table 1. Amino acid classification is threefold:
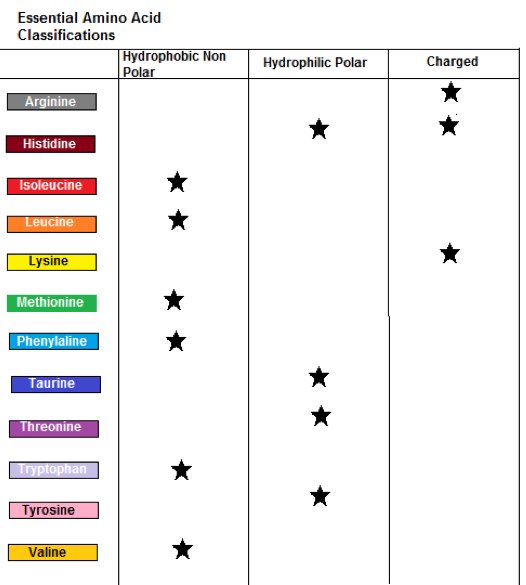
- Hydrophobic (non polar) or Water repellent which are used in the protein interior away from aqueous solution and in a more non polar fatty environment such as a component part of Cellular Lipid Bilayer (2 layers of fat) membrane surface.
- Charged means their elemental side chains are charged enabling these acidic amino acids can interact with polar amino acids by electrostatic attraction. The Charged amino acids are Glutamate and Aspartate -ve charged, while Arginine and Lysine and sometimes Histidine are +ve charged.
- Hydrophilic (Polar) or Water soluble used for proteins that are in contact with aqueous environments.
The following Table tabulates these distinctions:
Table 2: Amino acid Classification
Biological Purpose of Amino Acids
There exists some 500 Amino Acids but only 20 are used within the Mammalian Organism.
Aside from the manufacture of proteins from the genetic code, amino acids play other metabolic roles in the body.
Table 1 lists the general purposes of each essential amino acid.
Branched Chain Amino Acids (BCAA)

As shown in Table 1 there are 3 BCAAs Isoleucine, Leucine and Valine, which account for 35% of the total amino acid content in muscle tissue, and 40 % of the total amino acids used in mammalian physiology.
These 3 amino acids are normally associated with bodybuilding and muscle growth
Amino Acid Distribution

Amino Acid distribution in the body is complex, since proteins are made all of the time, every day, in addition to proteins that are taken in through the diet.
When proteins are consumed they are enzymatically hydrolyzed in the digestive tract, releasing Free individual amino acids that are absorbed by the gut.
Once absorbed, they are transported through the Hepatic Portal Vein to the liver where they are processed into glucose or fat or released into the bloodstream.
The tissues take up the amino acids for glucose production (Catabolic action), growth and biological maintenance (Anabolic action).
Normally, in an average body, there are approximately 55mg outside of the cells vs 800mg inside the cells.
In perspective the value of Free Amino Acids is a mere 1% compared to 99% that are used to make Proteins.
Protein/Amino Acid Basics
Protein/Amino Acid Storage
The body Recycles its own tissue protein to the tune of 100-300 grams.
The body does not store Protein or Amino Acids, although I suppose you could consider skeletal muscle acts like an Amino Acid store, since muscle tissue breaks down when exercising and the proteins are recycled by their Amino Acid constituents as I have just mentioned.
Futhermore, Muscle does not simply dump Amino Acids into the blood, but Branched Chain Amino Acids (BCAAs), as mentioned in the last section are metabolised for muscle growth, and the resulting metabolite is waste Nitrogen released into the blood as Alanine and Glutamine.
The former is transported to the liver as a precursor for Gluconeogenesis, a metabolic pathway that generates Glucose for cellular energy, and it + Glycine, Threonine, Cysteine, Serine and Trytophan make the Pyruvate module. The latter is used as precursor to make DNA
Naturally, as we sleep, we go into a fasting mode and again the body breaks down Protein to supply Amino Acids for the rest of the body.
The reason that Amino Acids are not stored in the body is because they are ‘Monomers’ (chemical building blocks), and they are soluble in water.
In order to store Proteins, all of the Amino Acid monomers would have to be available to make the proteins and store them.
The body uses some 50,000 Proteins.
To constantly replenish them using the Cellular Ribosome Protein Builders would need additional energy ( 4 *ATP/residue) on top of the daily requirement from gene expression.
In the same way, Glucose is soluble in water, but its storage form in the liver, Glycogen is not, since it is a ‘Polymer’ ( A collection of glucose monomer particles.).
This is how glucose is stored in the Adipose (Fat cells) tissue.
Why is solublility in water important in terms of storage ?, it is to do with Cellular Osmotic Pressure.
You may recall that Osmosis concerns the seperation of water by a semi-permeable membrane, permeable to water but not the solute inside the water ( This is how Desalination Plants can filter ocean water into drinking water by Reverse Osmosis).
Water moves from a high level to a low level due to osmosis, until both water levels on either side of the division ( in our case a cellular membrane) are equal and water flow stops, and an Osmotic Pressure has been created across the membrane.
In essence the more soluble a substance is, and if the body attempts to store it, an intolerable Osmotic pressure would be created causing cells to accumulate an excessive amount of water at the storage site causing Cellular Hypertonicity, where cells can literally swell and even burst.
Stress and Proteins
During stress excessive cortisol released by the Adrenals can cause the break down of proteins in the form of a loss of muscle tissue and Collagen, a scenario referred to as ‘Catabolic Response’, since Cortisol is a Catabolic Hormone.
Furthermore, a situation of Insulin Resistance (Diabetes), actually blocks the absorption of Amino Acids from the diet, and some could be the essential Amino Acids that the body cannot make.
This is why the Ketogenic Diet is so useful for Diabetics and individuals under stress.
The state of ketosis elevates Growth Hormone (GH) by 1300% in females and 2000% in Males which prevents and preserves protein loss, and stimulates Autophagy, which is the body’s mechanism to recycle Proteins and Amino Acids.
Growth Hormone(GH) is actually the opposing hormone to Cortisol, so when you are younger (20-30 for example), GH is ‘high so a little stress is successfully combated, but after 50 this hormone ‘bottoms out’, and you are no longer protected from the damaging affects from Cortisol due to long term stress.
Furthermore, Ketosis nomalises blood glucose and Insulin levels thus reversing Diabetes.
Thirdly is will reduce the requirement for dietary protein.
Recommended Daily Intake
The recommended protein intake for an adult should be approximately 0.8 * Body weight, so if you weigh 195 kgs then your protein intake should be :
0.8 * 195 = 156 grams/day ( 52grams/meal),
which is excessive (unless you are an extreme athelete that is burning a lot of protein).
The average person should be taking 0.4 * their body weight, so in our example this would equate to
0.4 * 195 = 78 grams/day (26grams/meal).
You can guage the amount of Protein that you consume in terms of the correct amount by your sleeping pattern.
Since Protein contains a lot of Phosphorous this acts as a stimulant, so if you suffer from lack of sleep then check your protein intake, presupposing that you are not stressed, since this will also cause a sleep disorder.
In the next section we will describe another physiological process that can occur due to abnormal dietary protein intake called ‘Deanimation’.
The Urea Cycle/Deamination
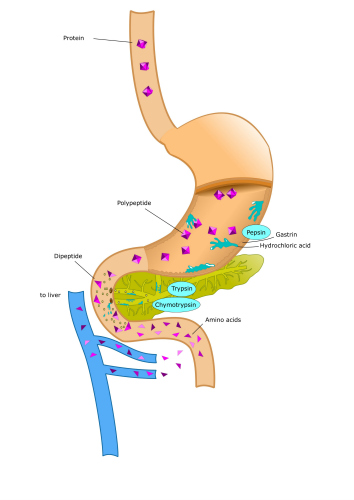
In situations where the body has excess protein the cells are unable to store the excess amino acids that constitute the protein, so the protein is degraded and hence the amino acids, have to be broken down by the liver.
However, the muscle cells have the ability to break down BCAAs Isoleucine, Leucine and Valine that we came across earlier.
The first step in amino acid degradation is is to remove the Alpha Amino Group via ‘Transamination’ forming Glutamate, achieved by an Enzyme Aminotransferase. (interestingly this enzyme needs a Vitamin B6 derivative coenzyme peridoxyl phosphate PLP that is used in cellular energy production in order for it to work).
The second step takes the Glutamate to form Ammonia NH3 and Carbon Dioxide.
This process is called ‘Deamination, a process that we came across in the microbiome articles dealing with disease, specifically stress where high cortisol levels can trigger this Deamination process in a negative way.
To further our discussion on stress, covered in the last section, high cortisol cause the dietary protein intake not to be absorbed into the muscle or bone (creating Osteoporosis) triggering the catabolic process by the liver to deanimate the amino acids into Ammonia and then excess Urea (Uric Acid).
Once the Ammonia has been removed, α–keto acids (Carbon Skeleton) remain that form carbohydrates that convert into glucose and the possibly into body fat, and due to Hydrogen ion (H+) and Potassium (K+) loss. this can make the body more alkaline, upsetting PH balance.
Why does the body behave in this way ?, since the dietary protein is not getting absorbed, the body senses a lack of muscle/bone replenishment, so as a protective (survival) mechanism, it generates extra adipose tissue (fat cells) to protect the organs.
This survival mechanism is also evident when the Adrenals are fatigued and body wants to hold on to body fat.
The body’s highest priority is survival even at the expense of how mishapen your body becomes. Its better to look like the Pilsbury dough man than suffering from ill health…lol.
Ammonia is highly toxic for the body and can cause death if allowed to accumulate.
The liver comes to the rescue, which activates the Urea cycle (one of the Methylation cycles) that consumes 2 molecules of Ammonia and 1 molecule of Carbon Dioxide, creating 1 molecule of Urea (NH2) 2CO which generates Ornithine (a proteinogenic amino acid) which activates the cycle for another turn.
The body cannot tolerate an accumulation of Urea either, but it is less poisonous than Ammonia, nethertheless, the urea is removed from the body by the kidney.
Ubiquitination of Proteins
I am not going into detail on this subject but just be aware that if proteins are damaged within a cell, the cell uses a 76 amino acid protein Ubiquitin to tag the damaged protein for amino acid breakdown.
This process involves 3 enzymes and 3 processes and goes beyond the discussion on essential amino acids.
Amino Acid Turnover
Amino acids when manufactured have variable life spans.
Proteins are continually being made and degraded at variable rates ( as discovered by Schoenheimer and Rittenberg in 1930), so enzymes that regulate proteins or peptide hormone signals have a high rate of synthesis/degradation and once their function is complete, the product is sent to the liver for breakdown and recycling.
On the other hand amino acids required for structural purposes such as Collagen, Myofibrillar (muscle fibres) Proteins and Plasma proteins have longer life spans.
Conclusion
In our opening statement we stated that amino acids provide life itself, and cells are protein producers using the amino acids in variable sequences that make the proteins.
Proteins that are synthesised within the body or are consumed through diet, all rely on additional nutrients that dictate their quality.
These nutrients are the Vitamins that are essentially activators from minerals that the body can’t possibly manufacture but rely on their availability from dietary consumption.
Through painstaking work of the great Dr Olree, theoretical geneticist and chiropractor has managed to associate the trace minerals to amino acids, and hence discover which individual amino acid was governed by which trace mineral.
Since the time of Buffon (French naturalist Georges Louis Leclerc de Buffon 1707-1788) and his book on natural history in 36 volumes beginning 1749 he states that the creator has not placed a fixed line between animal and vegetable and that these 2 species have many more common properties than real differences.
Buffon goes on to say that it appears very probable that there really exists in nature a number of small organized beings alike to the large organized bodies, and that these small organized beings are composed of living organic particles that are common to both animal and vegetable.
Unfortunately Buffon believed that the nutritional needs of mammals were simply to replace those substances (top up the natural reservoirs) that were lost through excretion and that the replacements were there only for augmentation (growth) and procreation and not as we know, to prevent physiological degradation, and as we also know, are the 90 essential nutrients that all humans should ingest daily.

“An amino acid residue (other than glycine) has no symmetry elements. The general operation of conversion of one residue of a single chain into a second residue equivalent to the first is accordingly a rotation about an axis accompanied by translation along the axis. Hence the only configurations for a chain compatible with our postulate of equivalence of the residues are helical configurations.”
Linus Pauling 1958
[Co-author with American chemist, ert B. Corey (1897-1971) and H. R. Branson]
Check out the Previous Article in this series:
https://www.extremehealthacademy.com/90-essential-nutrients-part-1-overview/
https://www.extremehealthacademy.com/90-essential-nutrients-part-2-b-vitamins-1-6/
https://www.extremehealthacademy.com/90-essential-nutrients-part-3-b-vitamins-7-12/
https://www.extremehealthacademy.com/90-essential-nutrients-part-4-vitamins-acde/
https://www.extremehealthacademy.com/90-essential-nutrients-vitamin-d/
https://www.extremehealthacademy.com/essential-nutrients-vitamin-e/
https://www.extremehealthacademy.com/essential-nutrients-vitamin-k/
https://www.extremehealthacademy.com/essential-nutrients-choline/
https://www.extremehealthacademy.com/essential-nutrients-part-9-flavonoidspolyphenols/
References/Acknowledgments:
- Proteins,essential amino acids BCAA, Monomer, Osmotic Pressure Wikipedia
- The Urea Cycle Kimball’s biology pages
- Dr Berg Videos on Protein Dr Eric Berg
- Hierarchical structure of proteins Book Molecular cell biology 1986 Baltimore, Lodish
- Introduction to Macromolecules Khan Academy
- Proteinstructures.com
- Modern Nutrition in health and disease M.Shils, M.Shike, C.Ross, B. Cabellero,R.Cousins
- Epigenetics Essential amino acids section Joel Wallach book 2014
- Natural History Georges Louis Leclerc de Buffon 1749
- Linus Pauling quote
- Why can’t amino acids be stored in the body? Mark Roseman Uniformed Services of Health Services Quora
Author: Eric Malouin