Introduction
Hopefully, readers of these articles are aware of the intelligent adaptive response of the human body to events that occur inside and outside of the body. Methylation happens millions of time/day in each of our cells in our lifetime that determines our destiny from one day to the next. The body is constantly adjusting and changing its internal environment based on its hosts dietary and lifestyle choices, taking into consideration the environment that the host is experiencing and the host’s response to his/her’s environment and experiences. Our modern way of living in general, is one of competition, which in some circumstances can have a deleterious effect on our bodies, whereas one of cooperation with others and world around us may have a more beneficial outcome to the condition of our bodies. Negative responses to the world around us causes negative outcomes within our bodies, such as arguments at work, heated disagreements with colleagues and friends, rage while driving. Whereas positive responses such as helping a friend through troubled times, providing money or shelter to a more unfortunate individual can provide a more physiologically positive result. A host that respects the needs of their bodies nutrient requirements will thrive, but the host that decides on dietary choices which promote nutrient deficiency such as processed and fast food will cause damage to the body and compromise the body’s ability to self heal and self regulate. Taking prescription drugs will cause a similar outcome as well as excessive toxic substances such as alcohol. The choices we make in life are continually monitored and adjusted accordingly by a physiological ‘Master’ process that is referred to as Methylation.
Methylation

Methylation involves the entire body, and its the body’s ‘global’ management of all physiological processes that maintain physiological and psychological balance. Some estimate that this process occurs a billions times/second…I wonder how they measured that ?, well at least we know it occurs continually many times/day. To do this, the body makes continual changes to the way we express our genes, and all of the physiological processes that control organ function. Enzymes, that we discussed in the previous article are methylated, so they work more efficiently, in fact it is enzymes that accomplish the methylation task, hormones and genes are all methylated to maintain efficient functioning. Methylating all the body’s proteins assist in the detox process. So how does the body methylate ?. Simply put, a chemical structure called a methyl group is bound to a protein which changes the way that proteins react to other substances in the body. However, methylation also involves removing a methyl group as well. It is the methylation process that takes the absorbed nutrients in the body and applies them to the cells that use them, for example to drive the energy production mechanism. In essence these methyl groups consisting of 1 carbon and 3 hydrogen atoms are transferred to a substrate (a molecule that is acted upon by an enzyme) that exists in DNA,RNA, Neurotransmitters, hormones, cells, for the purpose of accomplishing DNA,RNA synthesis and repair, gene regulation/epigenetic gene expression, neurotransmitter production, energy production, cell membrane repair, metabolism, myelination and immune function. The following diagram shows the biological mechanism that the body uses to accomplish its mammoth management task of methylation.
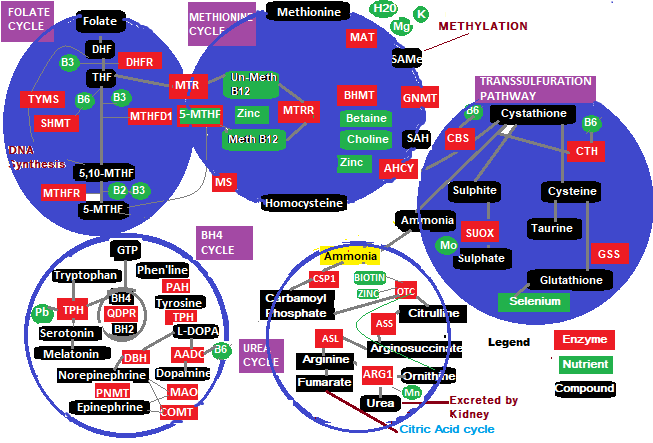
I know…it looks like a subway map, but let us discuss each cycle in turn. Cycles in blue background are the subject of discussion in this article. I have coloured the text in relation to the diagram so the descriptions are easier to follow, i.e RED for enzymes, GREEN for nutrients. I know this is a little technical but my aim is to emphasize the need for essential nutrients throughout the cycles.
Folate Cycle
The purpose of this cycle is to produce and donate a methyl group 5-MTHF (5-Methyltetrahydrofolate ) which is referred to as ‘Active folate, chemically bound to homocysteine to create methionine that occurs within the cytoplasm in the cell to keep the Methionine cycle going. An enzyme shown as MTR (5- Methyltetrahydrofolate-homocysteine methyltransferase or Methionine Synthase) makes the regeneration of methionine from homocysteine possible. Methionine synthase eventually becomes inactive due to the oxidation of cobalamin (B12) cofactor (methylcobalamin). Another enzyme shown as MTRR ( Methionine synthase reductase), is the reductase enzyme version of MTR that regenerates MTR. 5-MTHF is also called L-Methylfolate and is a B Vitamin in its own right. To initiate this cycle the body needs dietary folate which is a natural folic acid salt, water soluble Vitamin B9, and if you remember from the articles the’ 90 essential nutrients’, folate is crucial for pregnant mothers. Folate rich foods include legumes (Lentils, beans, peas), Asparagus, Leafy greens (Kale,Chard,Spinach etc), eggs, Beets, citrus fruits ( Lemons,Limes,oranges), brussels sprouts,broccoli. Once the folate has been hydrolyzed in the gut, for plasma transportation to the cells, DHF (Dihydrofolate ) is converted into THF (Tetrahydrofolate) by enzyme DHFR (Dihydrofolate reductase) using Vitamin B3 (Niacin) as its cofactor. A point to mention here, is that, natural folate from food must always be the dietary choice, despite the loss of folate due to harvesting, storage and heat. The synthetic version called Folic acid still has to be converted to a naturally bioactive form using the same DHFR enzyme, a slow process, since this enzyme is also required for the DHF to THF conversion, so there is a competitive issue, and not all of the folic acid is converted, leaving unmetabolized folic acid (UMFA) floating around. This UMFA floats in the blood plasma where some research has shown that it can reduce the efficiency of immune system natural killer cell lymphocytes in destroying cancer cells. The third issue is that folic acid is poorly absorbed in comparison to its natural counterpart.
Folate cycle purposes
Apart from delivering the methyl group 5-MTHF to bind with homocysteine, creating methionine, to maintain continuous operation of the Methionine cycle, it supplies a methyl group to the main methylator S-Adenosylmethionine ( SAMe). Since Bacteria and plants synthesise natural folic acid, a study that was conducted in 2011 showed that Lactobacillus Plantarum, is a strain found in some probiotics, including sauerkraut, sourdough bread, Nigerian Ogi ( A wet corn starch based pudding), Chinese sour mi fen noodles ( sweet potato based noodles), and Korean Kimchi. This hardy strain survives all the way through the intestinal tract into the stool, but it is not a permanent resident but while in residence, it vigorously attacks pathogenic strains, creating an environment hospitable for incubated resident colonies to expand. Apart from folate, it produces a number of antibiotics such as Lactolin, and the amino acid Lysine. This strain synthesises Folic acid in the presence of PABA ( Para-Aminobenzoic Acid), a compound found in eggs, grains, milk, molasses, liver and kidney. Bifidobacterium strains Adolescentis and Pseudocatenulatum also produce folate and are also used in some probiotics.
DNA Synthesis
As shown on the folate cycle part of the diagram TYMS (Thymidylate synthase ) is an enzyme used for monophosphate conversion, producing DTTP ( Deoxythymidine Triphosphate) which is a building block of DNA, as well, used to synthesize purines. Amino acid Serine is converted to Glycine and THF which is accomplished by SHMT (Serine Hydroxymethyltransferase) enzyme to produce 5.10 MTHF. Since this subprocess is responsible for DNA synthesis the drug companies have a field day tinkering with their poisons to manipulate the body, such as Methotrexate, a drug used to ‘treat’ leukemia and arthritis. This drug is an example of a folate antagonist, antimetabolite drug, that enters the folate cycle in preference to tetrahydrofolate and blocks the entire cycle. Folate deficiency ( and/or B12 deficiency) can have disastrous effects on many physiological processes and is behind conditions such as Megaloblastic anemia ( slowing down of red blood cell production), or affecting other fast replacement organs, such as the gut, causing malabsorption.
The role of MTHFD1 and MTHFR enzymes
The enzyme MTHFD1 ( Methylenetetrahydrofolate Dehydrogenase ) is involved in folate metabolism. It is fairly special since it is involved in 3 conversions of 5,10-MTHF, 5,10-methenylTHF and 10-formylTHF ( only the first is shown in the diagram). The conversion is shown on the diagram from THF to 5,10-MTHF that requires Pyridoxine ( Vitamin B6 ) and Vitamin B3 (Niacin). The final conversion uses MTHFR ( many people have heard of this enzyme due to its supposed common genetic mutation that can lead to high levels of homocysteine in the blood ??). The acronym MTHFR stands for Methylenetetrahydrofolate Reductase. As is shown in the diagram above, it converts 5,10 Methylenetetrahydrofolate (5.10 MTHF) to the methyl group donated to the Methionine cycle to 5-Methyltetrahydrofolate (5-MTHF). This conversion also requires nutrients Vitamin B3 (Niacin), and Riboflavin (Vitamin B2). We will discuss more about the panic, in the next article, that has many people reading all the hype about the myriad of diseases that can occur from this common mutation or transcription errors, causing people to have their genes tested. There is also substantial research concerning MTHFD1 ( Methylenetetrahydrofolate Dehydrogenase ) with respect to birth defects especially downs syndrome..blaming defective enzymes again. Yet another example of blaming faulty biological ‘parts’ from autosomal recessive origins, and even some studies have found that some individuals require more of a particular nutrient like choline to ‘stabilize’ the functioning of the enzyme, instead of reasoning that we are all biochemically individual. This means that some individuals need more of a particular nutrient than others, but this does not mean the gene that expresses the enzyme is faulty.??… chasing symptoms around and classifying them as the root cause.
The Methionine cycle
The Methionine cycle as shown in the diagram above, involves 4 substrates, Methionine, S-Adenosylmethionine (SAMe), S-Adenosylhomocysteine (SAH) and Homocysteine. This cycle is all about creating methyl groups. As in the folate cycle it needs dietary folate; in the methionine cycle, it requires dietary protein, and due to the interplay of these 2 cycles, the methionine cycle cannot initiate without the methyl group 5-MTHF being delivered from the folate cycle. The enzyme Methionine Adenosyltransferase ( MAT) and an ATP process converts Methionine into S-Adenosylmethionine (SAMe). Cofactors magnesium (Mg) and potassium (K+) are required nutrients for this enzyme to function. The enzyme Glycine N-Methyltransferase (GNMT) is used to regulate SAMe in response to development stages and metabolic changes, since SAMe, or its other name AdoMet, is involved in methylation for at least 50 different methyltransferases for cellular metabolisms, so regulation is important to maintain cellular homeostasis. Adenosylhomocysteine ( AHCY) converts S-Adenosylhomocysteine (SAH) to Homocysteine. Finally Methionine synthase (MS) converts Homocysteine to Methionine and the cycle repeats.
Homocysteine to Methionine conversion alternative pathway
BHMT (Betaine-Homocysteine S-MethylTransferase)
The BHMT Pathway uses Betaine,choline and zinc to convert Homocysteine to Methionine and is a pathway utilized by the body if Folate is deficient in the host, so it is an alternative methylation pathway to drive the main methyl donor SAMe. A study conducted in 2013 on African women showed that there was a seasonal switch between the folate and betaine pathways due to seasonal availability of dietary components. Betaine, an amino acid, also known as Trimethylglycine, is a rich substance found in Wheat bran, quinoa, wheat germ, Spinach, beets, and shellfish. Its health benefits include the promotion of muscle growth, to promote protein synthesis, to help the body process fats and protect cells under stress. It is a derivative of choline, which is a precursor, and must be present for betaine to be synthesized in the body. Betaine is produced from choline and another amino acid Glycine, and is considered a methyl donor.
No RDA has been established for Betaine, because ‘they’ are too busy applying dosage requirements for drugs….lol, but Betaine consumption varies between 100-400 mg/day ( 1 cup of raw beets = 129 mg, 1 cup of uncooked quinoa = 630mg, 1 cup of raw spinach = 550mg). The study showed low intake levels of betaine by rural African women at approx 33 mg/day. The BHMT pathway can actually produce Betaine by oxidising Choline via choline dehydrogenase and betaine aldehyde dehydrogenase. The betaine is then converted into Dimethylglycine (DMG) as it donates a methyl group to homocysteine, but of course a dietary source of choline ( red meat, poultry, milk, eggs and fish) must be present. An RDA value has been established for choline, which is, on average 500 mg/day. Since both the liver and kidney store large amounts of Betaine, the BHMT pathway is particularly active in these organs. Suffice to say that deficiency in all of these critical nutrients can lead to conditions pertaining to protein synthesis disorder in the liver and the buildup of excess fat in the liver as well as muscle dysruption. We mentioned above, how betaine can protect cells under stress, since Betaine is a major osmolyte in the cell, regulating cell volume and protein stability. When we discussed water as a nutrient in previous articles in this series, we discussed the relationship between the extracellular and intracellular fluid, and if an imbalance occurs, such that the extracellular fluid osmolarity exceeds that of the intracellular fluid, the cells experience hyperosmotic stress. Choline uptake is increased within the mitochondria which gets converted to Betaine, by oxidising choline, as explained above, to resist volume reduction and osmotic water loss. The benefits of Betaine have been known for 50 years or so within animal nutrition, especially the influence of lipid metabolism, which is enhanced by supporting the synthesis of carnitine, used to transport fatty acids to the mitochondria for energy production.
The transsulfuration pathway
The purpose of this pathway is to eliminate excess homocysteine, converting cysteine into amino acid taurine, sulfate and pyruvate, the conversion of sulfites to sulfates, the elimination of excess cysteine and producing and recycling Glutathione from Cysteine. Homocysteine is a crucial component of this pathway which is quite clear from the methylation diagram shown above. This pathway is regulated by the stimulation of enzyme Cystathionine B-synthase (CBS), and the inhibition of Methylenetetrahydrofolate Reductase (MTHFR), in response to changes in the level of S-Adenosylmethionine (SAMe), promoting the degradation of homocysteine when Methionine availability is high. Another nutrient is required, that assists in the production of sulphur based Glutathione, and that is Selenium, and deficiency in this mineral will increases transsulfuration of homocysteine and decrease global DNA methylation. Sulfite Oxidase (SUOX) is an enzyme resident in the mitochondria, that oxidises sulphite to sulphate. Sulphite is involved in the Cytochrome C complex in transferring electrons to allow ATP generation at the end stage of the ETC, and once this step is complete, the sulphur is metabolised, producing sulphate which is then excreted from the body. As is shown in the methylation diagram the SUOX enzyme needs mineral Molybdenum (Mo) as a cofactor to work.
Homocysteine transfer to the Transsulfuration pathway
If the body senses low glutathione levels it will divert some homocysteine to the Transsulfuration pathway which is intercepted by Cystathionine B-synthase (CBS) that converts homocysteine to cystathionine. From the diagram the active form of Pyridoxine ( Vitamin B6 ), called Pyridoxal Phosphate (PLP) is required as a cofactor for the CBS enzyme. Enzyme Cystathionine Gamma Lyase (CTH) converts cystathionine into cysteine. Interestingly, a by product from enzymatic reaction of CTH on cystathionine, is alpha Ketobutyric acid, which the body uses in the mitochondrial matrix, where it is converted to Propionyl-Coa, which ends up being converted to succinyl-CoA, an intermediate compound that is part of the citric acid cycle, and activated during the Complex II stage of the electron Transport chain. Ammonia is also produced by the CTH activation that is delivered to the Urea cycle that converts this toxic product into urea and excreted out of the body through the urine. We will cover the citric acid cycle in a future article. The CBS enzyme is not found in the heart, lung, teste, adrenal and spleen suggesting that these organs have increased sensitivity to homocysteine toxicity, and L-Cysteine must come from outside these organs.
Conventional medicine’s wild goose chase looking for defective enzymes ( not genes this time)
Medical science believe that the overexpression of CBS and a low level of homocysteine in the blood is the major culprit behind ‘Downs syndrome’, resulting in the creation of CBS inhibitors drugs, despite the fact that, according to Dr Joel Wallach, Trisomy ( Down’s syndrome), characterized by 47 chromosomes instead of 46, is not caused by a hereditary defect but a maternal nutrient deficiency. The story goes that in 1997, Lauren Knievel, the niece in law of Evel Knievel, was 2 ½ months pregnant and her embryo was diagnosed with Downs syndrome and it was recommended to abort ( remember during embryonic growth the first 3 months is crucial in building the neonate and the other 6 months is simply growth of the final ‘product’). Wallach had only 15 days to reverse the situation by applying all 90 essential nutrients which actually worked. I might add Wallach was a little skeptical because the time window to reverse the ‘genetic defect’ may have already passed. I cannot imagine the untold damage that would be inflicted if these CBS inhibitors drugs were brought to market ( at least they were not, up to Nov 2016 ref: https://www.ncbi.nlm.nih.gov/pubmed/27521834 ) in terms of interfering with the entry point of the methylation ‘Transsulfuration pathway’.
Gasotransmitters


Of course the most common gaseous substance for humans is oxygen, which, apart from keeping us alive, has signalling properties as well as in most organs, including the mitochondria’s signalling through the ETC. There are 3 known functional ‘gasotransmitters Nitric oxide (Nitrogen Monoxide), Hydrogen Sulphide and Carbon Monoxide.
Endothelial Nitric oxide synthase (eNOS) I
eNOS is an enzyme particularly associated with the cardiovascular system since the Nitric oxide produced by eNOS in the vascular endothelium regulates vascular tone, cellular proliferation, leukocyte adhesion and platelet aggregation. eNOS requires a crucial cofactor Tetrahydrobiopterin ( also known as Sapropterin or BH4) to synthesise Nitric oxide and without it eNOS produces Superoxide, a very damaging free radical that we discussed previously, produced in the Mitochondria from leaking oxygen out of the ETC. BH4 we will discuss in the next article dealing with the remaining methylation cycles BH4 and Urea. There exists a pathway termed Nitrate-Nitrite-Nitric oxide which is initiated by nitrate (NO3) rich foods such as green leafy vegetables and beets. When consumed, commensal bacteria that reside in the mouth reduce the nitrate content to nitrite (NO2) which the body then converts into nitric oxide (NO), which has an effect of increasing circulating plasma and enhancing cardiovascular performance, and increasing oxygen flow to various organs including the heart, which is ideal for exercise. Furthermore, from the previous articles on the Microbiome we explained how the immune system Phagocytes use nitric oxide as a secreted free radicals weapon which is toxic to bacteria and parasites
Hydrogen Sulphide (H2S) – Synaptic plasticity
This gas which the body uses as a signalling molecule ( the other 2 are Nitric oxide as described in the previous paragraph and Carbon Monoxide), is produced from cysteine by enzymes Cystathionine B-synthase (CBS) and Cystathionine Gamma Lyase (CTH). It provides relaxation of smooth muscle and vasodilation of blood vessels. A glutamate receptor in the brain NMDA (N-methyl-D-aspartate) found in nerve cells, responds to hydrogen sulphide as a signalling mechanism to control synaptic plasticity. Synaptic plasticity refers to the brain’s ability to adapt to new input circumstances by adjusting the sensitivity settings on nerve cell synapse junctions likened to a volume control that is adjusted for maximum efficiency when nerve cells are communicating with each other. Long term synaptic plasticity is the dominant model applied to how the brain stores information, measured in time ( minutes/hours/days/years ) as memory storage. Psychologists refer to short term and long term memory and a rehearsal buffer, so if you wanted to force the brain’s memory storage to remember something and transport it to long term memory storage you would repeat read something or repeat a series of word(s) over and over, or even listen to song lyrics over and over again. These repetitive exercises is part of the rehearsal buffer and once the repetitive action is complete, hopefully, it delivers the information from short term memory into long term memory where the brain makes the necessary biological adjustment to the relevant nerve cell synapses and the information remains in long term memory storage.
Hydrogen Sulphide (H2S) – Metabolic and body temperature regulation
Since both Hydrogen sulphide and nitric oxide both relax blood vessels it is postulated that Nitric oxide effects the larger venous vessels, and Hydrogen sulfide provides the benefit in the smaller blood vessels and capillaries, although it is quite possible that they are interchangeable. Both these gases provide stimulatory signals in the blood, enhancing blood flow, for example providing penile erection ( no more viagra pills…just hook yourself up to a gas line…lol). Researchers have postulated that the natural production of hydrogen sulphide is used in mammals to regulate metabolism and body temperature by binding to cytochrome oxidase, the enzymatic complex in Complex IV stage of the mitochondria, inhibiting the enzyme. With cytochrome oxidase coupled with higher concentrations of molecular oxygen, it slows down the metabolic rate and lowers body temperature ( to the brown adipose tissue ), the mechanism that drives the hibernation state in some animals; a state referred to as clinical Torpor. In 2005 it was shown that mice could be placed into a state of suspended animation like hypothermia by applying low doses of hydrogen sulphide causing the animals breathing rate to change from 120 to 10 breaths/minute and their body temperature dropped from 37 C to 2 C. After 6 hours of this procedure the mice survived without any after effects.
Conclusions
I have attempted to describe three of the methylation cycles, Folate, Methionine and Transsulfuration, on an overview level, since there is more going on that we probably do not understand, but suffice to appreciate this biological series of interprocesses need particular essential nutrients for it to function efficiently. There is much discussion around concerning the mutancy or transcription errors that are common in some of the genes that express the many enzymes involved, which we will discuss later. In the next article we will describe the other remaining two methylation cycles BH4 and Urea and explain DNA methylation.
Check out other Articles in this series:
Nutrients in Food and their bodily purpose I (Phenols)
Nutrients in Food and their bodily purpose II (Lignans, Triterpenes, Phytosterols, Carotenoids & Fats)
Nutrients in Food and their bodily purpose III (Phenolic acids, sulphur, sulphides,sulphoxides )
Nutrients in Food and their bodily purpose IV (Glucosinolates, Sulforaphane, Indole-3-Carbinol)
Nutrients in Food and their bodily purpose V (Lipid distribution, absorbed fats, Criciferous Veg)
Nutrients in Food and their bodily purpose VI (Nutrients required for Liver Detox)
Nutrients in Food and their bodily purpose VII (Seeds & the Omega Fatty Acids)
Nutrients in Food and their bodily purpose VIII (Nutrients required for cellular energy production)
Nutrients in Food and their bodily purpose IX (Water I Properties and Body fluids)
Nutrients in Food and their bodily purpose X (Water II Cellular Hydration)
Nutrients in Food and their bodily purpose XI (Water III Fluid filtration, reabsorption, excretion)
Nutrients in Food and their bodily purpose XII (Water IV Blood pressure, Blood volume regulation)
Nutrients in Food and their bodily purpose XIII (Water V Body Fluid Dysfunction
Nutrients in Food and their bodily purpose XIV (Dental Nutrients)
Nutrients in Food and their bodily purpose XV (Nutrients involved in Methylation I)
Nutrients in Food and their bodily purpose XVII (Nutrients involved in Methylation III)
Nutrients in Food and their bodily purpose XVIII (Nutrients involved in Methylation IV)
Nutrients in Food and their bodily purpose XIX (Methylation V and the Microbiota I)
Nutrients in Food and their bodily purpose XX (Methylation VI and the Microbiota II)
Nutrients in Food and their bodily purpose XXI (Superfoods: Wheatgrass)
Nutrients in Food and their bodily purpose XXII (Superfoods: Adaptogens)
Nutrients in Food and their bodily purpose XXIII (A look into our nutritional past Sir Robert McCarrison)
Nutrients in Food and their bodily purpose XXIV (Pregnancy: Nature vs Nurture vs Nutrition)
References/Acknowledgments :
- Overview of Homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects Blom & Smulders 2011 NCBI ( PubMed)
- Probiotics 2016 Case Adams
- Methylation report sample Lifecode Gx
- Folate Production by probiotic bacteria 2011 Maddalena Rossi at al NCBI (PubMed)
- Folate cycle Herbaltransitions.com
- Rodak’s Hematology Clinical principles and applications Keohane,Smith & Walenga 2016
- Glycine N-Methyltransferase and regulation of S-Adenosylmethionine Levels 2009 Luka,Mudd & Wagner NCBI (PubMed)
- What is Betaine ? 2015 Jillian Levy Dr Axe
- Foods highest in Betaine SELFNutritionData
- The Metabolic burden of methyl donor deficiency with focus of the betaine homocysteine methyltransferase pathway 2013 Rima Obeid NCBI (PubMed)
- Tetrahydrobiopterin, Endothelial NOS, biological functions of Nitric oxide, Cystathionine beta synthase, Gaseous signalling molecules,NMDA Receptor Wikipedia
- The nitrate-nitrite-nitric oxide pathway: its role in human exercise physiology 2012 Stephen Bailey et al European Journal of sport medicine
- What is synaptic plasticity University of Queensland
Author: Eric Malouin