Introduction
In this article we are going to look at prostaglandins and the drugs that are used to interfere with their natural purpose and in one particular case, people were killed for the sake of trying to artificially reduce inflammation by disturbing crucial biological pathways.
It is of the utmost importance that you appreciate humans with so few number of genes and so few amino acids, we function by using the few amino acids in thousands of protein sequences metabolised in thousands of different pathways, many shared pathways that define our infinitely complex being, so drug scientists are never going to design the perfect drug to interfere with our biology without serious consequences as you can appreciate from the diagram below:
Interesting, now you can understand that drug manufacture can never be that successful because attempting to block anything in the body blocks hundreds of other processes and hundreds of shared pathways, doing untold and unknown potential damage and malfunction, even by a simple mechanism like pain relief.
Remember there are no ‘quick fixes’ without consequences and in some cases dire consequences.
In this article we relate the unfortunate circumstances that evolved over the drug Vioxx produced by Merck and a look at one illegal clinical trial that was conducted by Pfizer on foreign soil.
Prostaglandins and COX Inhibition:
(The Vioxx Debacle)

Introduction to Prostaglandins
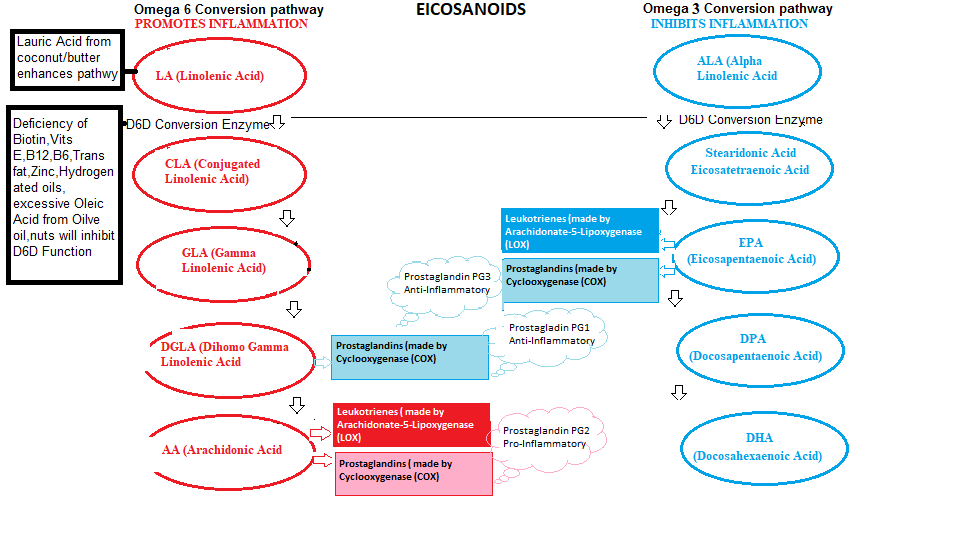
If you have read the articles on Metabolic typing, I explained the importance of balancing Prostaglandins (as shown in the recreated diagram below).
Prostaglandins behave in a similar manner as endocrine hormones but they are Autocrine/Paracrine derived (acting on itself/acting within the vicinity or localised).
Prostaglandins are a member of a family of substances called Eicosanoids, which are manufactured by a dietary intake of Polyunsaturated Fatty Acids (PUFA).
Prostaglandins are transient, insofar as they are metabolised rapidly.
Prostaglandins, which are essential to health, specifically protect from heart disease such as Atherosclerosis (prostaglandins are essential signaling molecules for Vasodilation or relaxation of the heart muscle).
PG3 series Prostaglandins are responsible for protecting the body from heart attack and also acting as a mediator for Inflammatory response.
The Omega 6 Conversion pathway promotes Inflammation (as it has to repair the body) and the Omega 3 Conversion Pathway inhibits Inflammation (once repair is complete).
‘PGs’ provide Anti-Clotting functions, Lowering of Triglycerides (fats in the blood), Blood Pressure control and the Improvement of Skin conditions.
You will notice also from the diagram certain Nutrient Deficiencies ( Biotin and Vitamins E,B12,B6) actually inhibit both functional pathways, because it inhibits D6D (Delta 6 Desaturase) conversion enzyme.
Polyunsaturated Fatty Acids (PUFA) are dietary derived where Omega 6 fatty acids in the form of Linoleic Acid (LA) contained in Vegetable oils, nuts and advocadoes, and Omega 3 Fatty acids in the form Alpha Linoleic Acid (ALA) contained in Flax.Hemp and Chia seeds, Grains and Green vegetables.
In terms of Prostaglandin production, the Omega 6 conversion pathway‘s purpose is to produce dihomo-γ-linolenic acid (DGLA), and Arachidonic Acid (ARA), while the Omega 3 conversion pathway is to produce Eicosapentaenoic acid (EPA).
Prostaglandins are the Body’s messenger ‘hormones’ that deal with injury and dysfunction, so prostaglandins are produced on demand at locations where there is tissue damage or infection, and are part of the inflammation process.
For example, if a blood vessel is injured, a Prostaglandin called ‘Thromboxane’ is produced to form a blood clotting function to heal the damage, and in turn restricts the blood flow to reduce blood loss by contracting the blood vessel wall.
If the body needs to reduce blood clotting and remove blood clots after damage repair Prostaglandin ‘Prostacyclin’ is generated for this purpose, as well as relaxing the blood vessel wall.
Prostaglandins are not produced by one particlar gland as are hormones, every organ in the body can produce them, and they are regulated by 2 enzymes Cyclooxygenase-1 (COX1) and Cyclooxygenase-2 (COX2).
COX1 provides normal prostaglandin production in a healthy body, but if dysfunction occurs COX2 swings into action producing additional prostaglandin products.
As can be seen from the diagram below, the biological circuitry is there to maintain balance within the body like Yin and Yang, where the Omega 6 pathways promote inflammation to induce the healing process, while the Omega 3 pathways inhibit inflammation when the body completes the healing process. So PG series 1 and 3 are anti-inflammatory products, PG series 2 is inflammatory.
This same circuitry is also involved in Leukotriene production which are also Eicosanoids inflammatory mediators involved in the smooth muscle of the bronchia for example.
Another observation is the recommended 2:1 ratio which is necessary since if you swing too far in either direction.
Typical ratio for people eating fast food or processed food could by 15 or 20:1, which would continuously saturate the conversion enzymes, and leave nothing to activate the Omega 3 conversion pathway, unless you consumed enough fish oil and/or fish eggs.
In effect you have opposing systems to maintain balance synonymous to the ANS Sympathetic (On switch ) and the Parasympathetic (Off switch).
The Pharmaceutical company scientists have designed Anti-inflammatory drugs, such as Aspirin and Ibuprofen, to block the action of the Cyclooxygenase enzymes and so reduce Prostaglandin levels.
Foolishly they work against the body’s natural Eicosanoid process believing that it is helpful to relieve the symptoms of inflammation.
Similarly Aspirin blocks the production of thromboxane preventing unwanted blood clotting in patients with heart disease.
The next chapter describes the disaster that occurred when a COX2 inhibitor was introduced into the market, a drug class referred to as a NSAID (Non steroidal anti-inflammatory drug).
The VIOXX (Rofecoxib) Story: Chapter 1

A drug VIOXX defined as a NSAID (Non steroidal anti-inflammatory drug) COX2 * inhibitor,whose purpose was to treat symptoms of Osteoarthritis (degenerative joint disease) and Primary Dysmenorrhea (cramping in the lower abdomen before and during menstruation).
It was Nov 1998 when Merck pharmaceuticals approached the FDA requesting approval for Vioxx after testing the new product on 5.400 subjects in 8 studies.
It touted its new drug as superior to older painkillers since it caused less gastrointestinal problems.
In fact the trial which was referred to as the Advantage trial which did include some 5000 patients and 600 doctors, but the trial was actually a ‘seeding trial’.
Seeding trials are not designed to verify the efficacy and safety of a drug but it is project driven by marketing to promote and make aware of the product to as many doctors as possible, in order for them to come familiar with handling its prescription profile, so at some stage in the future they would prescribe the drug.
In 2008 when documents pertaining to ADVANTAGE came to light, it confirmed that the trial was a marketing objective where marketing handled the scientific and marketing data including the collection and dissemination.
Merck failed to reveal the true nature of the trial to participants, physician investigators and the institutional review board members, and as is the case with seeding trials it was simply a drug promotion project.
* COX (Cyclooxygenase) is an enzyme that initiates the formation of prostaglandins (as discussed above). It also produces thromboxane which is a vasoconstrictor that regulates arterial blood pressure by constricting blood vessels to prevent excessive blood loss by platelet aggregation to stimulate clotting when an injury occurs. It is the arachidonic acid from the diet ( as shown in the diagram) that synthesizes Thromboxane (TxA) and Prostacyclin (PG2) and there is a delicate balance between these 2 compounds to either inhibit vasoconstriction or platelet aggregation which is why COX2 Inhibitors cause Thrombosis (and Vioxx had the same effect) due to the the drug’s interference with the balancing process.
The VIOXX (Rofecoxib) Story: Chapter 2
In January, 1999 Merck launched another trial VIGOR (Vioxx Gastrointestinal Outcomes research study) including 8000 participants .
The object of the trial was to compare Vioxx against Naproxen (an older painkiller) in terms of their respective safety on the digestive system.
In May 1999, the drug was approved by the FDA, and in Oct 1999 the first meeting of VIGOR’s DSMB (Data and safety monitoring board) convened, concluding that Vioxx use resulted in fewer stomach ulcers and gastrointestinal bleeding than Naproxen.
Then in Nov 1999 the DSMB met again and confirmed that as of Nov 1, out of 4000 Vioxx taking patients, 79 had died compared to 41 taking Naproxen and the minutes reflected this trend reporting that
‘While the trends are disconcerting,the numbers of events are small’.
The panel then voted to continue the study and meet again the following month.
WHAT?…
EXCUSE ME..the trends are disconcerting…..Your drug has just killed 79 people, and 41 people from Naproxen not to speak of the Thrombosis that it causes.
Even today this poison is still an approved prescription drug, despite the fact that, I quote from the drugs.com website :
Naproxen can increase your risk of fatal heart attack or stroke, especially if you use it long term or take high doses, or if you have heart disease. Even people without heart disease or risk factors could have a stroke or heart attack while taking this medicine.
The purpose of both these drugs is to treat Osteoarthritis and pre-menstrual cramps..SO WHY WOULD ANYBODY OF SOUND MIND WANT TO RISK HAVING A HEART ATTACK OR THROMBOSIS WHEN BOTH THESE CONDITIONS CAN BE TREATED IN A NON TOXIC NATURAL WAY.
So when the DSMB met again in Dec 1999 they confirmed that the risk of serious heart problems and death for Vioxx taking patients is twice that of Naproxen taking patients, and still they signed off as a quorum to continue with the drug, defending its position by saying that:
‘We couldn’t tell if Vioxx was causing the heart problems or if naproxen, acting like low-dose aspirin, protected people from them, making Vioxx to look risky by comparison’….
If you had read the pathology it is quite clear whats causing the heart problems..BOTH, including Aspirin which is also a NSAID.
The DSMB did suggest that Merck devise an analysis of the study into the cardiovascular results before the study ended, which Merck rejected.
Two members of the DSMB wanted an immediate analysis done.
At least a month before the last patient left the study in February 2000, Merck and the DSMB agreed to analyze the heart problems, which in my opinion would have taken all of 15 minutes because as I explained above :
Prostaglandins, which are essential to health, specifically protection from heart disease such as atherosclerosis (prostaglandins are essential signaling molecules for Vasodilation or relaxation of the heart muscle). PG3 series Prostaglandins are responsible for protecting the body from heart attack. The thrombosis as explained above is caused by the interference of PG2 (Prostaglandin 2) and TxA (Thromboxane) biological balancing process between and initiation/ inhibition of a clotting factor.
There, “the proof is evident that these drugs are dangerous and you are risking your ass by taking them, in the same way you would in fighting an alligator with your bare hands.”
The VIOXX (Rofecoxib) Story: Chapter 3
In March, 2000 Merck received the results from the VIGOR study which were not complimentary toward their drug.
So to cut a longer story short, between Jan 2002 to Aug 2004 numerous epidemiological studies confirmed increased cardiovascular risk associated with taking Vioxx, and by Sept 2004 Merck pulled the plug on Vioxx, but by now 20 million US citizens had taken the drug.
Research later published in the Lancet uncovered that 88,000 had heart attacks and 38,000 had died. Merck ended up by paying $4.85 billion to end the thousands of law suits filed against Vioxx.
Closing statement
It is evident that the Allopathic medical establishment have convinced the masses that Inflammation is bad for the body since they regularly prescribe NSAIDs (Non steroidal anti-inflammatory drugs), in an attempt to reduce inflammation. I quote the eminent Dr John Bergman :
“If you work against the body, you move closer to death, if you work with the body, you move closer to life”
This a very profound statement, since, in this case it refers to artificially suppressing inflammation, which is working against the body, and not allowing the body to follow its natural inflammatory response which is working with the body.
So take heed, all who think otherwise :
” Inflammation must be allowed to occur since it is a natural part of our healing process from damage”
The Trovan Antibiotic Study: Chapter 1
In 1996 in Kano Nigeria a Meningitis epidemic broke out which Pfizer Pharmaceuticals took advantage of, by conducting a randomized trial using an experimental drug Trovafloxacin a broad spectrum Antibiotic.
The purpose of the study was to compare Trovafloxacin (Trovan) with a competitive Antibiotic Ceftriaxone, a fungal derived Cephalosprin which was already approved and had been proven to be effective.
Unfortunately, the participants were not informed of the experimental drug treatment that was injected into 200 infected children ( 100 received Ceftriaxone, and 100 received Trovan).
Futhermore, Pfizer deliberately gave a reduced dose of Ceftriaxone presumably ( as was the allegation ), to make their new drug Trovan appear to work better. Although, Pfizer admitted to reducing the dosage of Ceftriaxone for reasons of minimizing the injection site pain.
The dosage given to the children of Ceftriaxone was 33mg/kg despite the recommended dose of between 50-100mg.kg by the Clinical trail organisers Medicin Sans Frontieres
What transpired after the 2 Antiobiotics were administered was a disaster which killed 11 children (6 who were given Ceftriaxone and 5 from Trovan).
Other children were blinded, deafened and brain damaged.
It was also alleged that this was an illegal drug trial that was not sanctioned by the Nigerian government or even consented by the childrens parents.
However, the lead Nigerian investigator produced a letter of approval to conduct human trials, which was proven to be falsified.
This begs the question, why did Nigerian officials allocate to Pfizer two hospital wards to conduct the testing ??.
It was also revealed that despite Pfizer’s protocol to secure parental consent were faced with very few parents that could speak or even write English, and that Pfizer did not explain that the proposed treatment was experimental and they were quite within their rights to refuse it, and to be told that alternative proven treatments were available.
These were some of the facts that came out of a law suit action that was filed by Nigerian parents initially in 2002 against Pfizer and heard in a New York district court.
It was also discovered that animal testing of Trovan performed prior to this disaster indicated that significant side effects toward human children were found to indicate that the drug may cause joint disease, abnormal cartilage growth (Osteochondrosis) and liver damage.
The Nigerian Plaintiffs also alleged that Pfizer failed to conduct their protocol of blood testing the selected children upon arrival and 5 days after to evaulate the efficacy of the drug administered, and then to swap drugs if it was found negative.
The Plaintiff’s also claimed that low dose administration of Ceftriaxone led to the subsequent injuries and death.
After the official approval from the FDA was given to Pfizer in February 1998, authorising its use for adults, the FDA and Pfizer began receiving reports of patients suffering from Liver damage
In January 1999, the FDA changed its approval criteria for Trovan use only for patients with life threatening illnesses, and the FDA then issued a public health advisory notice regarding Trovan and its asssociation with Liver Toxicity and post marketing reports regarding high risk acute liver failure.
After several Judicial opinions dismissing the case on procedural grounds which involved Pfizer arguing that informed consent for experimental drug trials in Africa were not required, and any legal issues should be heard in Nigeria, the legal battle dragged on.
In 2002 WHO ( World Health Organisation) submitted a report describing the Military government of Nigeria who were in power at the time “pervasively corrupt” and who knows what background arrangements were made between the Nigerian government and Pfizer
In May 2006 Senator Tom Lantos for California got involved when he got hold of a report documenting the proceeding of the case and immediately demanded Pfizer open its records, and then finally those Nigerian families, the 200 Plaintiffs, got their justice when in 2009 Pfizer settled out of court to the tune of $75 million between itself and the Plaintiff’s representative, the Kano State government of Nigeria
What also came out was that “Pfizer had hired investigators to uncover corruption links to federal attorney general Michael Aondoakaa to expose him and put pressure on him to drop the federal cases.” as reported by Wikileaked US embassy cables
In addition, the medical records of the victims from the 1996 Trovan Drug trials mysteriously got lost, an announcement made after the Nigerian State government received $10 million from the Pfizer settlement of $75 million.
Meningitis Pathogen : Neisseria Meningitidis
For those of you who are unaware of the source of Meningitis, it is produced by a pathogenic bacterium exclusive to humans and a very nasty pathogen called Neisseria meningitidis which is actually a virus which was originally isolated in 1884 when it was found inside cells within the cerebral spinal fluid.
As you might remember from previous articles I quote :
‘A virus is a prolific parasite that has no means to replicate since they are not a cellular organism; they usurp a eukaryote species of bacteria and use their resources to reproduce their off spring’.
In general, a virus is a mere 30 gene species, but they possess an ingenious ‘Cloaking device’ in order to latch on to their targets.
Once attached, the virus injects its own DNA into the host cell interior, forcing the cell to make copies of itself and any necessary proteins.
However, if the host bacteria contains ‘Restrictive Enzymes’ they can cut up the foreign DNA preventing replication.
The fascinating part of these enzymes is, they have the ability to recognize specific DNA sequences that they want to remove and then rejoin the spliced ‘sticky ends’ in a similar manner to the old audio tape splicing made contiguous for playback.
Restrictive enzymes cannot be used to protect the Mammalian Human Cell
If Mammalian cells had restrictive enzymes it would be the perfect method of protection particularly against the nasty Neisseria meningitidis virus but we don’t, we do however possess a virus defense mechanism called rNA silencing (this is reserved for another article).
The human also relies on Immune system antibody production to combat viruses.
Bacteria can use Endonucleases or restrictive enzymes because bacteria when they methylate they add a methyl group to Adenine residue, but viruses do not, so it is easier for the bacterial species being attacked by the virus to distinguish the virus DNA to its own DNA.
Humans, on the other hand methylate Cytosine residue, meaning that if humans did use restrictive enzymes, the enzymes would end up cutting up the human DNA as well as the virus DNA.
The Trovan Antibiotic Study: Closure

This case was exposed in 2000 by an investigation by the Washington Post (Watergate fame).
In 2002 and 2005 the victims filed a series of unsuccessful lawsuits in the US, until in 2009 a US court of appeals overturned the previous unsuccessful lawsuits and allowed the families to sue Pfizer under the ‘Alien Tort Statute and in July 2009 Pfizer agreed to pay $75 million to the state of Kano.
It was also discovered that Trovan was also strongly associated with 14 cases of acute live failure.
John le Carre’s novel and a subsequent movie ‘The constant Gardener ‘ was made, based on the true events of this case.
It’s like it’s a marriage of convenience and all it produces are dead offspring.
- Sorry, I’ve just got one question: Whose map is Britain using when it completely ignores the United Nations and decides to invade Iraq? Or do you think it’s more diplomatic to bend to the will of a superpower and politely take part in Vietnam the Sequel?
- They’re a drug company, Arnold. Come on, no drug company does something for nothing.
Movie quote The Constant Gardener
Check out the Previous Article in this series:
https://www.extremehealthacademy.com/selling-sickness-part-1-conventional-medicine-is-a-business/
https://www.extremehealthacademy.com/selling-sickness-part-2-profits-before-health/
https://www.extremehealthacademy.com/selling-sickness-part-3-baffling-the-masses/
References/Acknowledgments:
- Bad pharma how drug companies mislead doctors and harm patients Book 2013 Ben Goldacre
- Vioxx the downfall of a drug Nov 2007 Prakash & Valentine npr.org
- Naproxen drugs,com
- Abdhullahi vs Pfizer Wikipedia
- Deadly medicines and organised crime book 2013 Peter Gotzche
- Travofloxin, Neisseria meningitidis, cyclooxygenase, Prostaglandins Wikipedia
- Prostaglandins You and your hormones
- Series 1 Prostaglandins Health products Distributors Inc
- Quote from the movie ‘the constant gardener‘ 2001 IMDB
Author: Eric Malouin